Function and Pathway Enrichment Analysis of Corrmotifs (Concentration)
Last updated: 2025-06-06
Checks: 6 1
Knit directory: CX5461_Project/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of
the R Markdown file created these results, you’ll want to first commit
it to the Git repo. If you’re still working on the analysis, you can
ignore this warning. When you’re finished, you can run
wflow_publish to commit the R Markdown file and build the
HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20250129) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 305a6c0. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .RData
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: 0.1 box.svg
Ignored: Rplot04.svg
Untracked files:
Untracked: 0.1 density.svg
Untracked: 0.1.emf
Untracked: 0.1.svg
Untracked: 0.5 box.svg
Untracked: 0.5 density.svg
Untracked: 0.5.svg
Untracked: Additional/
Untracked: Autosome factors.svg
Untracked: CX_5461_Pattern_Genes_24hr.csv
Untracked: CX_5461_Pattern_Genes_3hr.csv
Untracked: Cell viability box plot.svg
Untracked: DEG GO terms.svg
Untracked: DNA damage associated GO terms.svg
Untracked: DRC1.svg
Untracked: Figure 1.jpeg
Untracked: Figure 1.pdf
Untracked: Figure_CM_Purity.pdf
Untracked: G Quadruplex DEGs.svg
Untracked: PC2 Vs PC3 Autosome.svg
Untracked: PCA autosome.svg
Untracked: Rplot 18.svg
Untracked: Rplot.svg
Untracked: Rplot01.svg
Untracked: Rplot02.svg
Untracked: Rplot03.svg
Untracked: Rplot05.svg
Untracked: Rplot06.svg
Untracked: Rplot07.svg
Untracked: Rplot08.jpeg
Untracked: Rplot08.svg
Untracked: Rplot09.svg
Untracked: Rplot10.svg
Untracked: Rplot11.svg
Untracked: Rplot12.svg
Untracked: Rplot13.svg
Untracked: Rplot14.svg
Untracked: Rplot15.svg
Untracked: Rplot16.svg
Untracked: Rplot17.svg
Untracked: Rplot18.svg
Untracked: Rplot19.svg
Untracked: Rplot20.svg
Untracked: Rplot21.svg
Untracked: Rplot22.svg
Untracked: Rplot23.svg
Untracked: Rplot24.svg
Untracked: TOP2B.bed
Untracked: TS HPA (Violin).svg
Untracked: TS HPA.svg
Untracked: TS_HA.svg
Untracked: TS_HV.svg
Untracked: Violin HA.svg
Untracked: Violin HV (CX vs DOX).svg
Untracked: Violin HV.svg
Untracked: data/AF.csv
Untracked: data/AF_Mapped.csv
Untracked: data/AF_genes.csv
Untracked: data/Annotated_DOX_Gene_Table.csv
Untracked: data/BP/
Untracked: data/CAD_genes.csv
Untracked: data/Cardiotox.csv
Untracked: data/Cardiotox_mapped.csv
Untracked: data/Corrmotif_GO/
Untracked: data/DOX_Vald.csv
Untracked: data/DOX_Vald_Mapped.csv
Untracked: data/DOX_alt.csv
Untracked: data/Entrez_Cardiotox.csv
Untracked: data/Entrez_Cardiotox_Mapped.csv
Untracked: data/GWAS.xlsx
Untracked: data/GWAS_SNPs.bed
Untracked: data/HF.csv
Untracked: data/HF_Mapped.csv
Untracked: data/HF_genes.csv
Untracked: data/Hypertension_genes.csv
Untracked: data/MI_genes.csv
Untracked: data/P53_Target_mapped.csv
Untracked: data/Sample_annotated.csv
Untracked: data/Samples.csv
Untracked: data/Samples.xlsx
Untracked: data/TOP2A.bed
Untracked: data/TOP2A_target.csv
Untracked: data/TOP2A_target_lit.csv
Untracked: data/TOP2A_target_lit_mapped.csv
Untracked: data/TOP2A_target_mapped.csv
Untracked: data/TOP2B.bed
Untracked: data/TOP2B_target.csv
Untracked: data/TOP2B_target_heatmap.csv
Untracked: data/TOP2B_target_heatmap_mapped.csv
Untracked: data/TOP2B_target_mapped.csv
Untracked: data/TS.csv
Untracked: data/TS_HPA.csv
Untracked: data/TS_HPA_mapped.csv
Untracked: data/Toptable_CX_0.1_24.csv
Untracked: data/Toptable_CX_0.1_3.csv
Untracked: data/Toptable_CX_0.1_48.csv
Untracked: data/Toptable_CX_0.5_24.csv
Untracked: data/Toptable_CX_0.5_3.csv
Untracked: data/Toptable_CX_0.5_48.csv
Untracked: data/Toptable_DOX_0.1_24.csv
Untracked: data/Toptable_DOX_0.1_3.csv
Untracked: data/Toptable_DOX_0.1_48.csv
Untracked: data/Toptable_DOX_0.5_24.csv
Untracked: data/Toptable_DOX_0.5_3.csv
Untracked: data/Toptable_DOX_0.5_48.csv
Untracked: data/count.tsv
Untracked: data/ts_data_mapped
Untracked: results/
Untracked: run_bedtools.bat
Unstaged changes:
Deleted: analysis/Actox.Rmd
Modified: analysis/Corrmotif_Conc.Rmd
Modified: analysis/Corrmotif_Conc_GO.Rmd
Modified: data/DOX_0.5_48 (Combined).csv
Modified: data/Human_Heart_Genes.csv
Modified: data/Total_number_of_Mapped_reads_by_Individuals.csv
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/Corrmotif_Conc_GO.Rmd) and
HTML (docs/Corrmotif_Conc_GO.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | df65b82 | sayanpaul01 | 2025-06-04 | Commit |
| html | df65b82 | sayanpaul01 | 2025-06-04 | Commit |
| Rmd | cf7130e | sayanpaul01 | 2025-06-03 | Commit |
| html | cf7130e | sayanpaul01 | 2025-06-03 | Commit |
| Rmd | c5f10c4 | sayanpaul01 | 2025-06-02 | Commit |
| html | c5f10c4 | sayanpaul01 | 2025-06-02 | Commit |
| Rmd | c034ee0 | sayanpaul01 | 2025-06-02 | Commit |
| html | c034ee0 | sayanpaul01 | 2025-06-02 | Commit |
| Rmd | cf95313 | sayanpaul01 | 2025-03-10 | Commit |
| html | cf95313 | sayanpaul01 | 2025-03-10 | Commit |
| Rmd | 11895c8 | sayanpaul01 | 2025-02-27 | Commit |
| html | 11895c8 | sayanpaul01 | 2025-02-27 | Commit |
| Rmd | 6484005 | sayanpaul01 | 2025-02-27 | Commit |
| html | 6484005 | sayanpaul01 | 2025-02-27 | Commit |
| Rmd | 87137ac | sayanpaul01 | 2025-02-27 | Commit |
| html | 87137ac | sayanpaul01 | 2025-02-27 | Commit |
📌 Load Required Libraries
library(tidyverse)
library(ggfortify)
library(ggplot2)
library(cluster)
library(edgeR)
library(limma)
library(Homo.sapiens)
library(BiocParallel)
library(qvalue)
library(pheatmap)
library(clusterProfiler)
library(AnnotationDbi)
library(org.Hs.eg.db)
library(RColorBrewer)
library(readr)
library(TxDb.Hsapiens.UCSC.hg38.knownGene)
library(DOSE)
# Load UCSC transcript database
txdb <- TxDb.Hsapiens.UCSC.hg38.knownGene📌 0.1 Micromolar
📌 Read and Process Data
# Load the saved datasets
prob_1_0.1 <- read.csv("data/prob_1_0.1.csv")$Entrez_ID
prob_2_0.1 <- read.csv("data/prob_2_0.1.csv")$Entrez_ID
prob_3_0.1 <- read.csv("data/prob_3_0.1.csv")$Entrez_ID
prob_1_0.5 <- read.csv("data/prob_1_0.5.csv")$Entrez_ID
prob_2_0.5 <- read.csv("data/prob_2_0.5.csv")$Entrez_ID
prob_3_0.5 <- read.csv("data/prob_3_0.5.csv")$Entrez_ID
prob_4_0.5 <- read.csv("data/prob_4_0.5.csv")$Entrez_ID
prob_5_0.5 <- read.csv("data/prob_5_0.5.csv")$Entrez_ID
CX_0.1_3 <- read.csv("data/DEGs/Toptable_CX_0.1_3.csv")
background<-as.character(CX_0.1_3$Entrez_ID)📌 Non response (0.1 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Libraries
library(tidyverse)
library(patchwork)Warning: package 'patchwork' was built under R version 4.3.3library(data.table)Warning: package 'data.table' was built under R version 4.3.3# 📁 Load File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_Non_response_(0.1).csv")
# 🧠 Convert and filter (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# Create tibbles for each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plot function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🔧 Plot for each GO category
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine plots
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot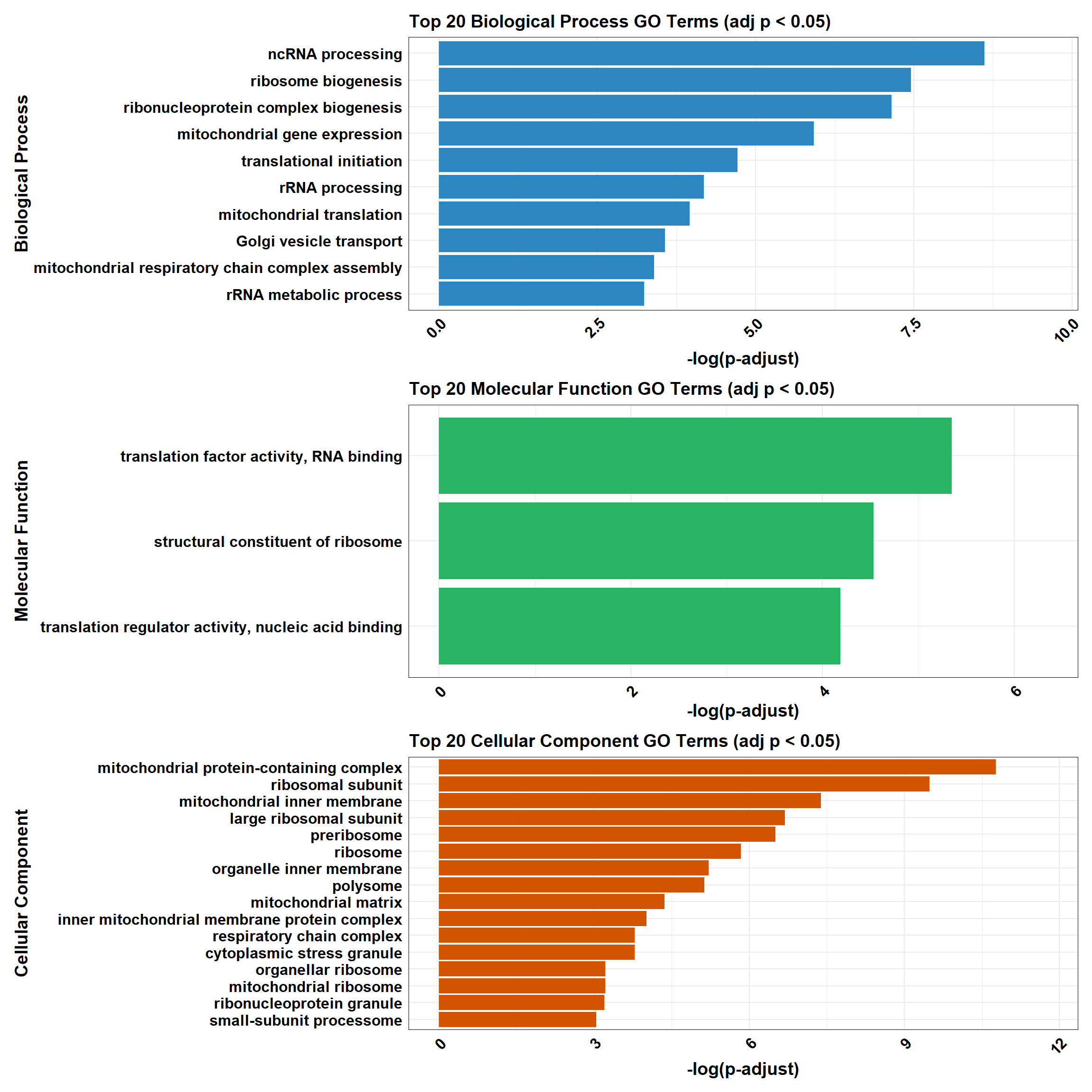
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)Warning: package 'gprofiler2' was built under R version 4.3.3library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_1_0.1, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}Warning: The `size` argument of `element_rect()` is deprecated as of ggplot2 3.4.0.
ℹ Please use the `linewidth` argument instead.
This warning is displayed once every 8 hours.
Call `lifecycle::last_lifecycle_warnings()` to see where this warning was
generated.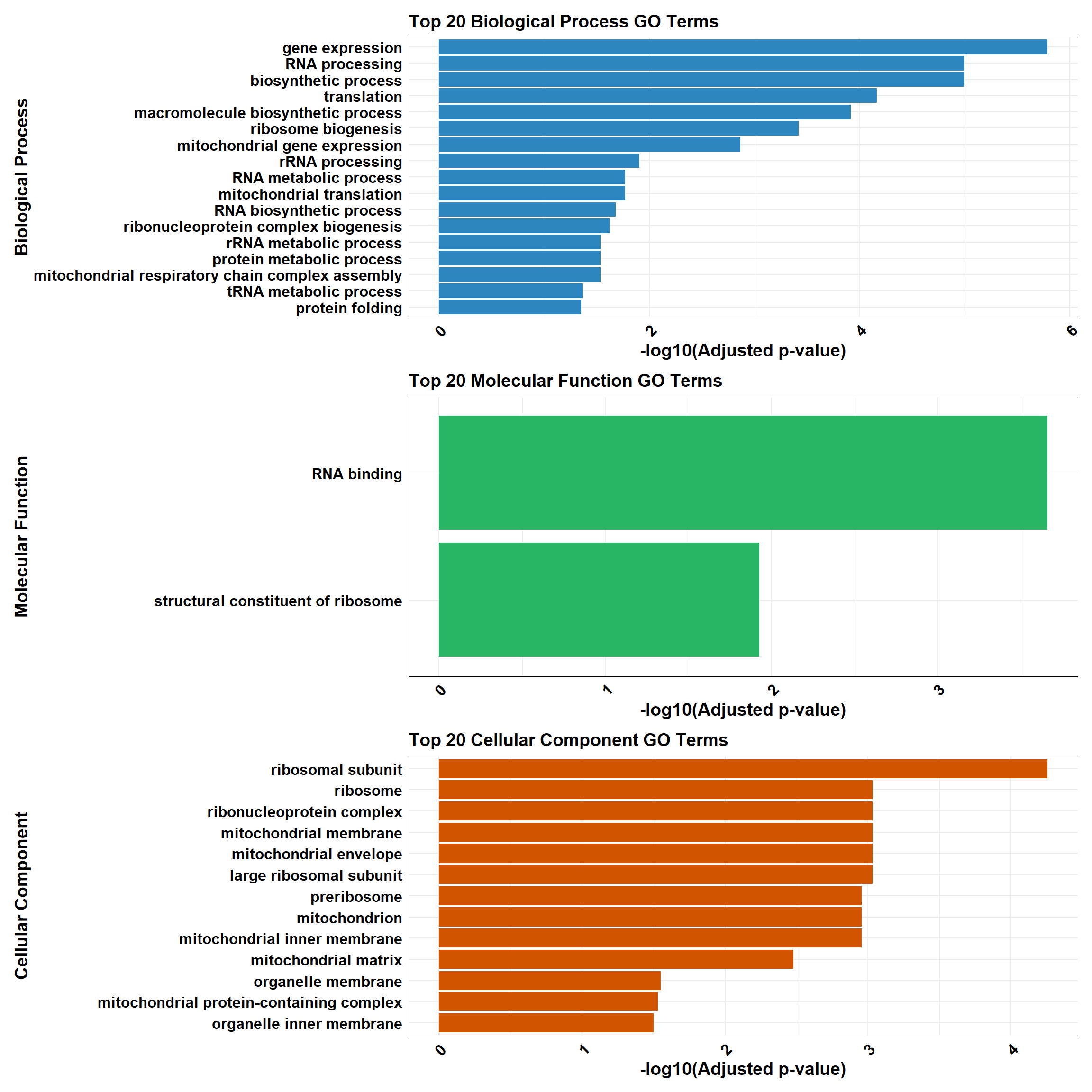
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)Warning: package 'ReactomePA' was built under R version 4.3.1# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_1_0.1,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_1_0.1,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_1_0.1,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_1_0.1,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)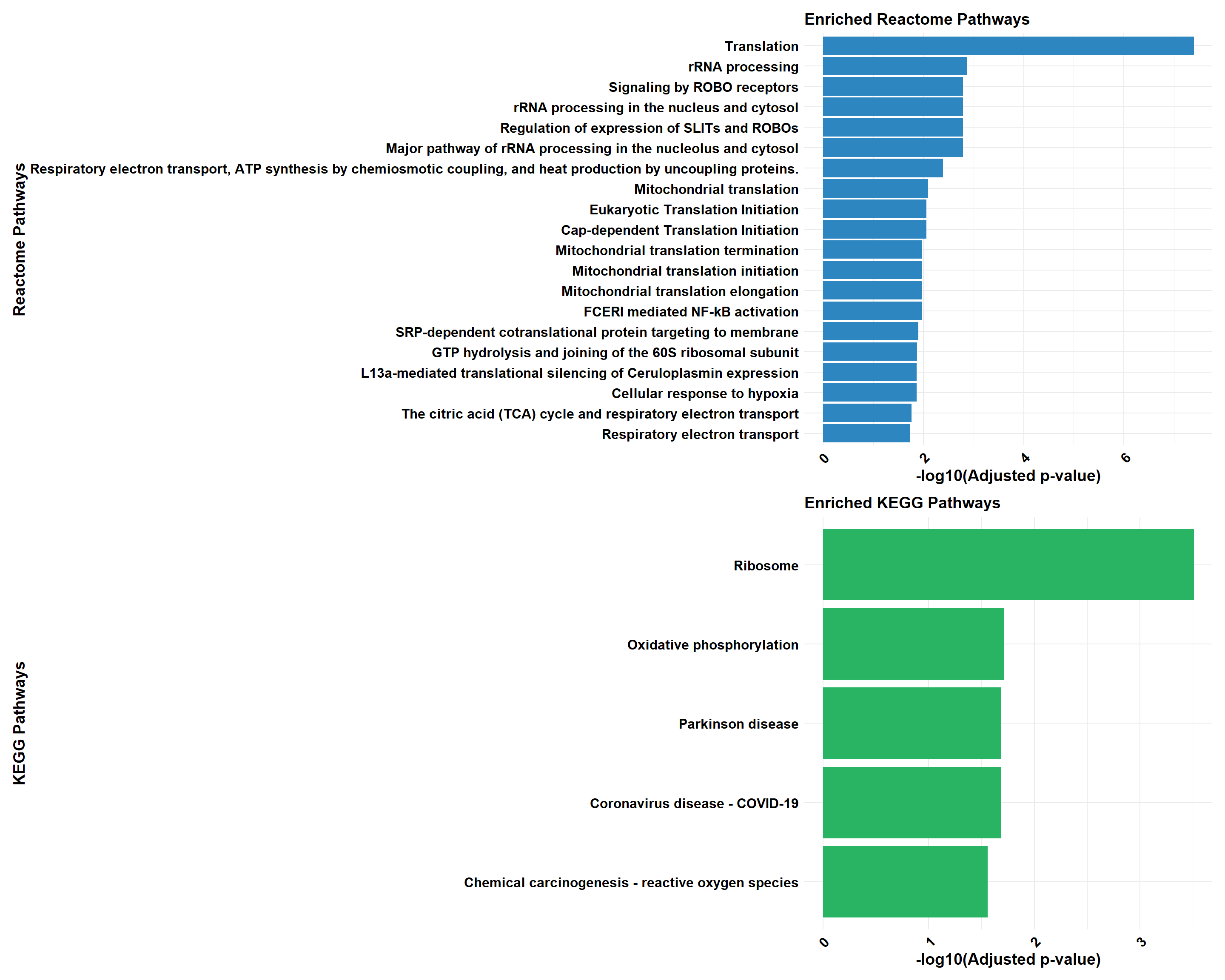
if (!is.null(gprofiler_combined)) print(gprofiler_combined)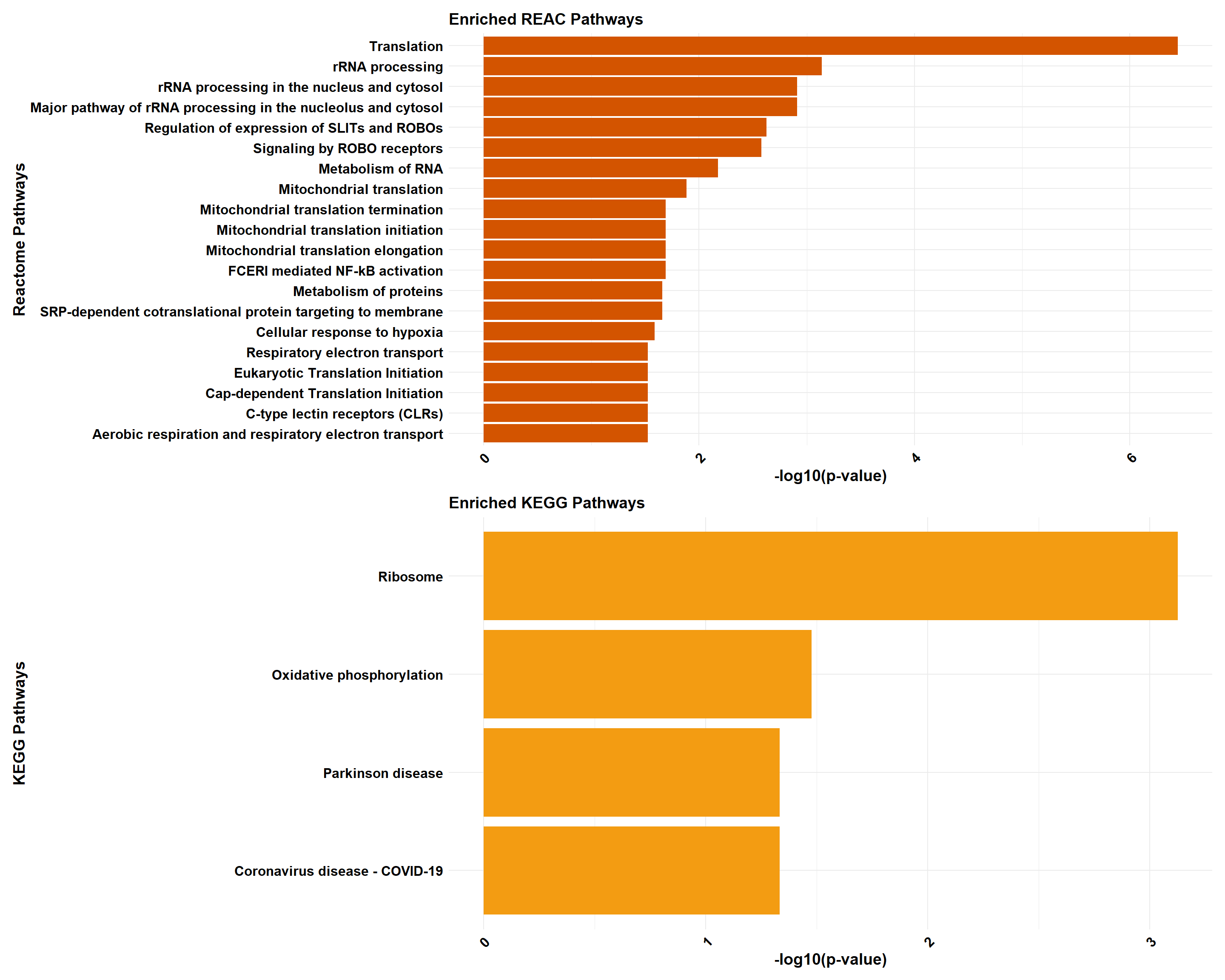
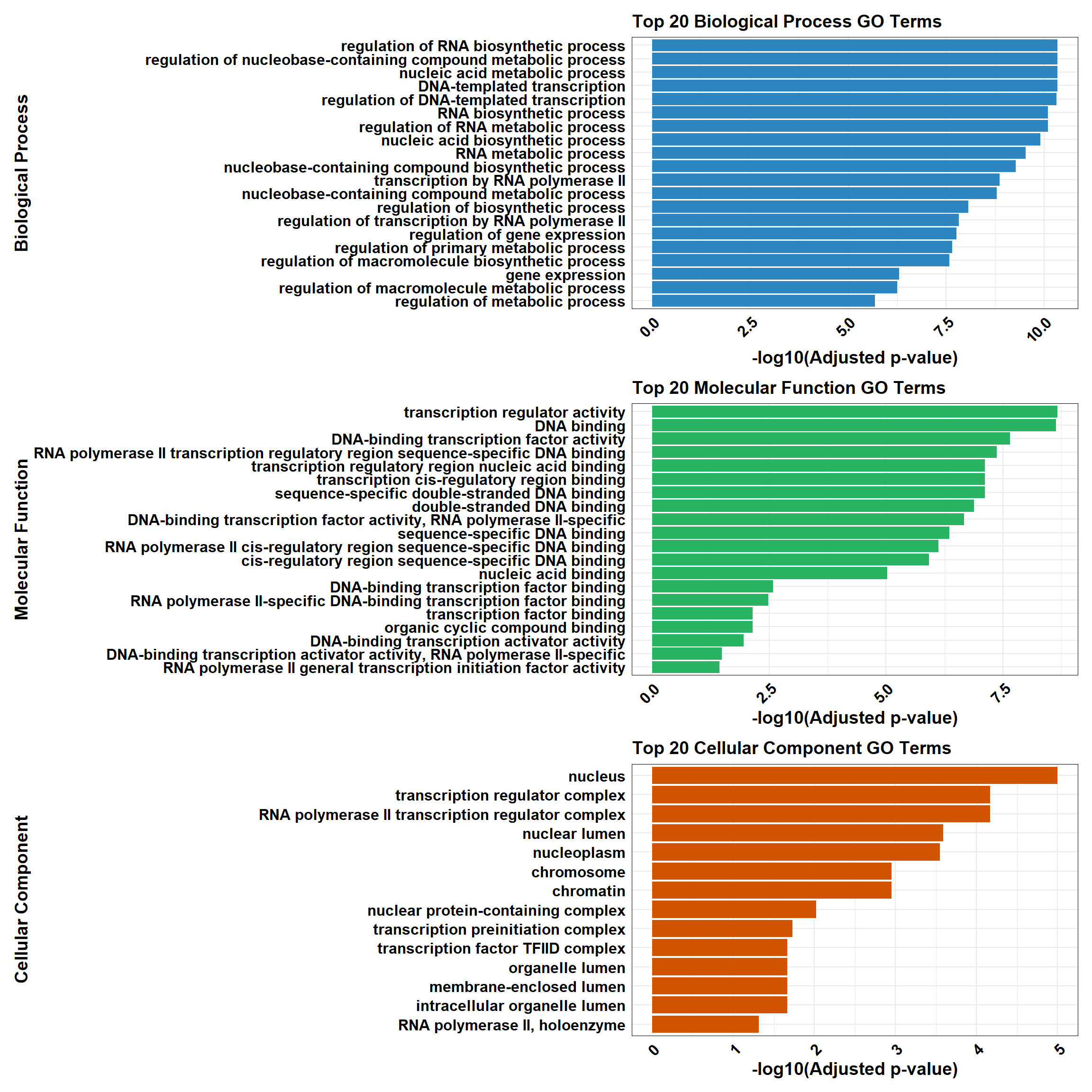
📌 CX-DOX mid-late response (0.1 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load GO CSV for CX-DOX mid-late response (0.1)
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_CX-DOX_mid-late_response_(0.1).csv")
# 🧠 Convert and filter (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# Create tibbles for each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plot function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🔧 Plot for each GO category
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine plots
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot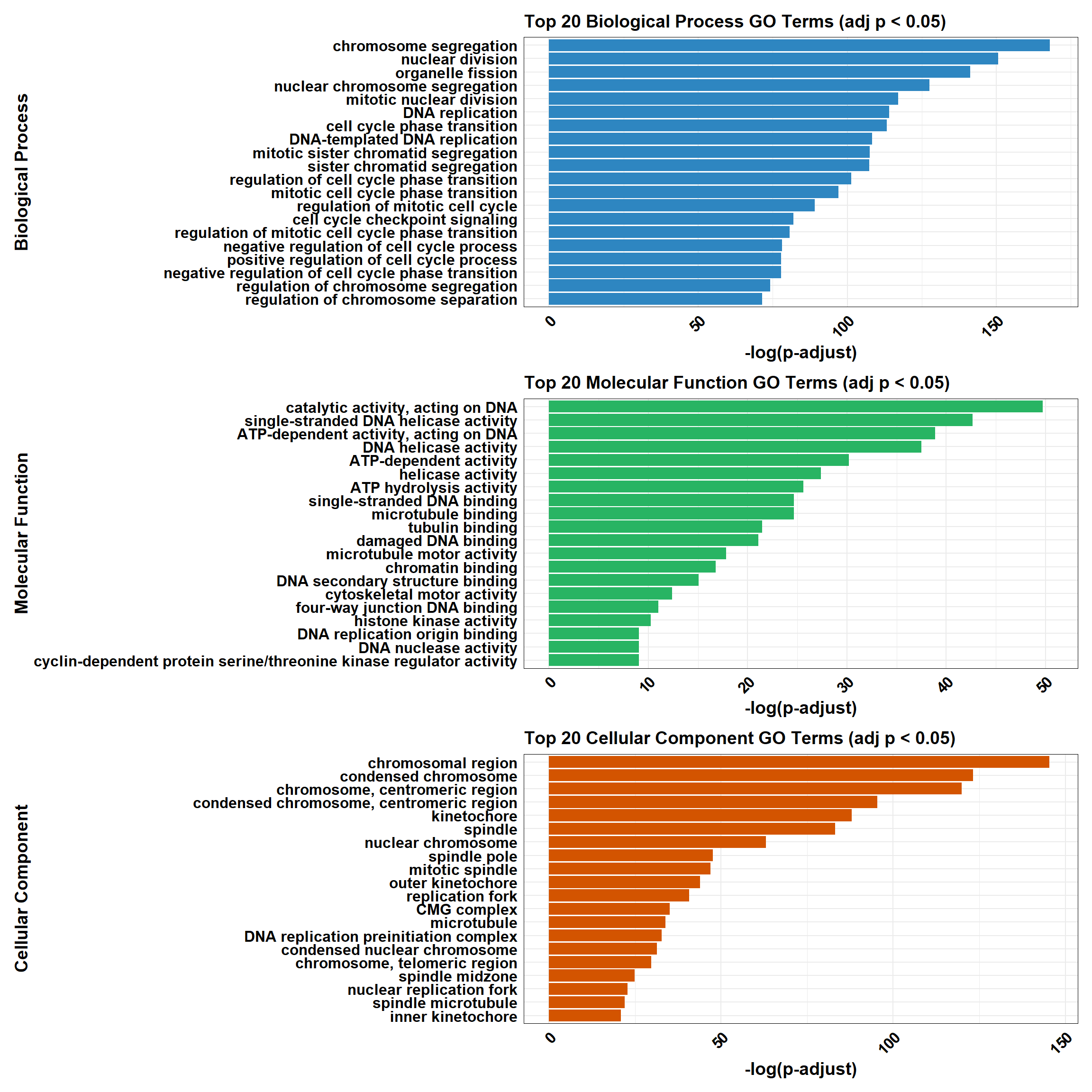
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_2_0.1,
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
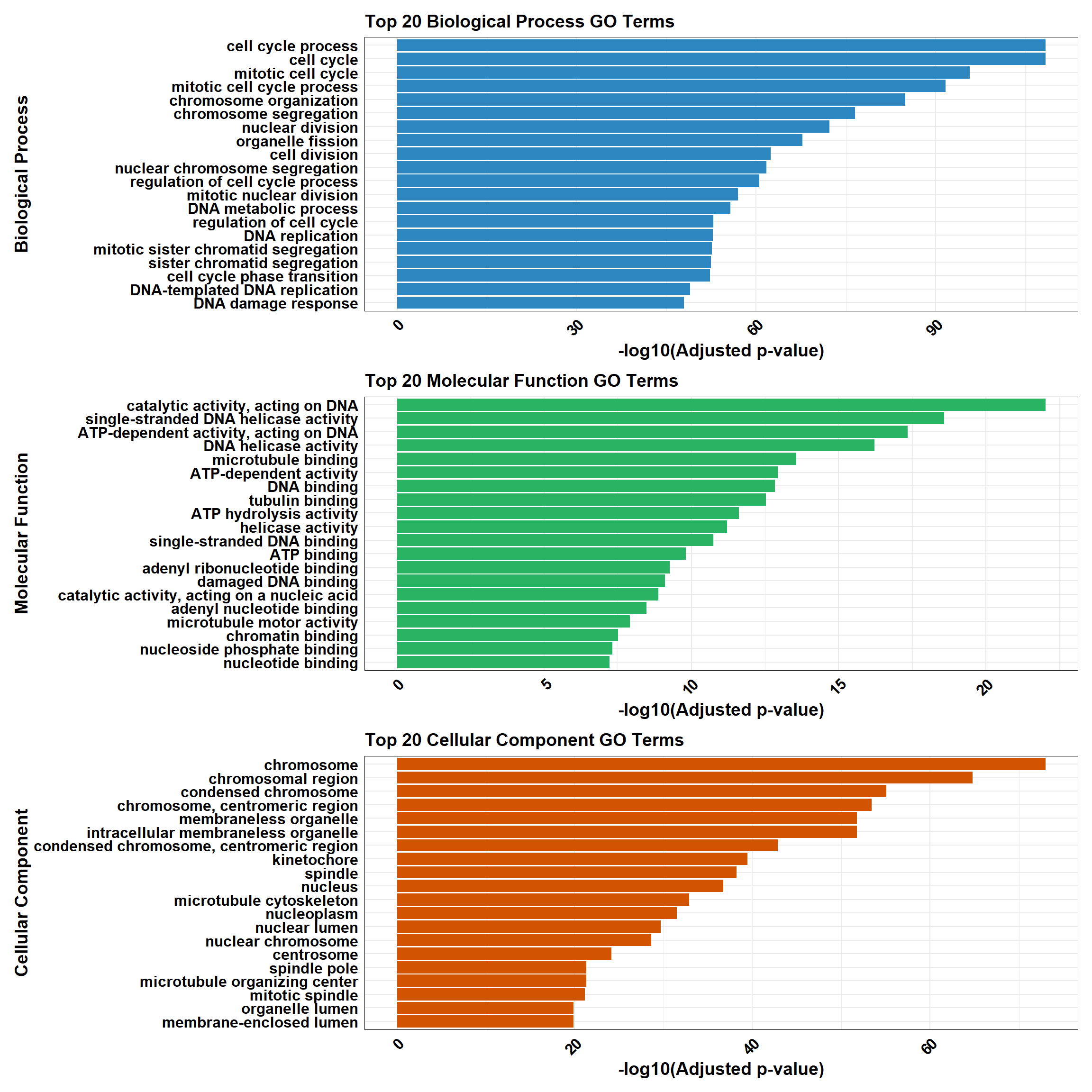
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_2_0.1,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_2_0.1,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_2_0.1,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_2_0.1,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)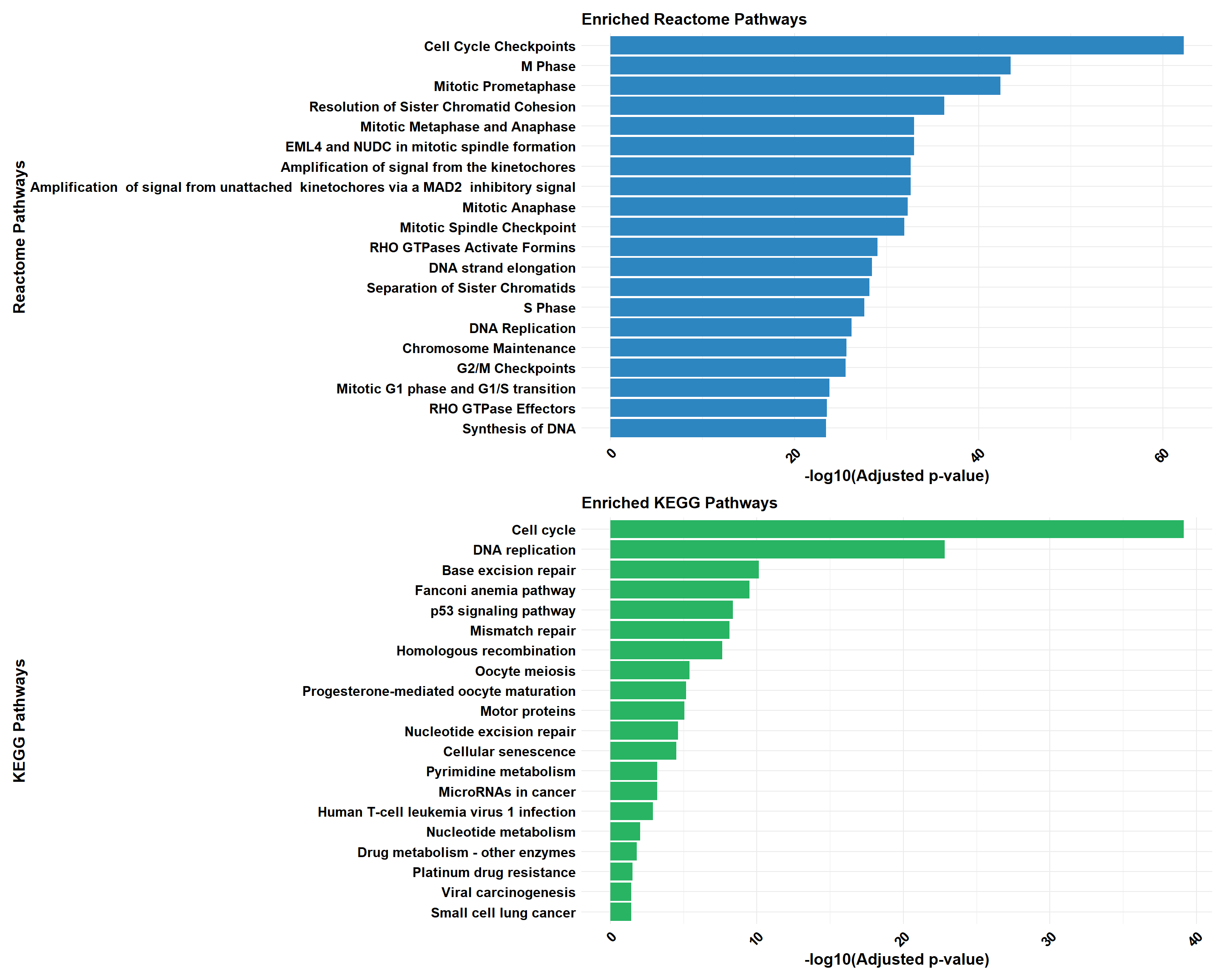
if (!is.null(gprofiler_combined)) print(gprofiler_combined)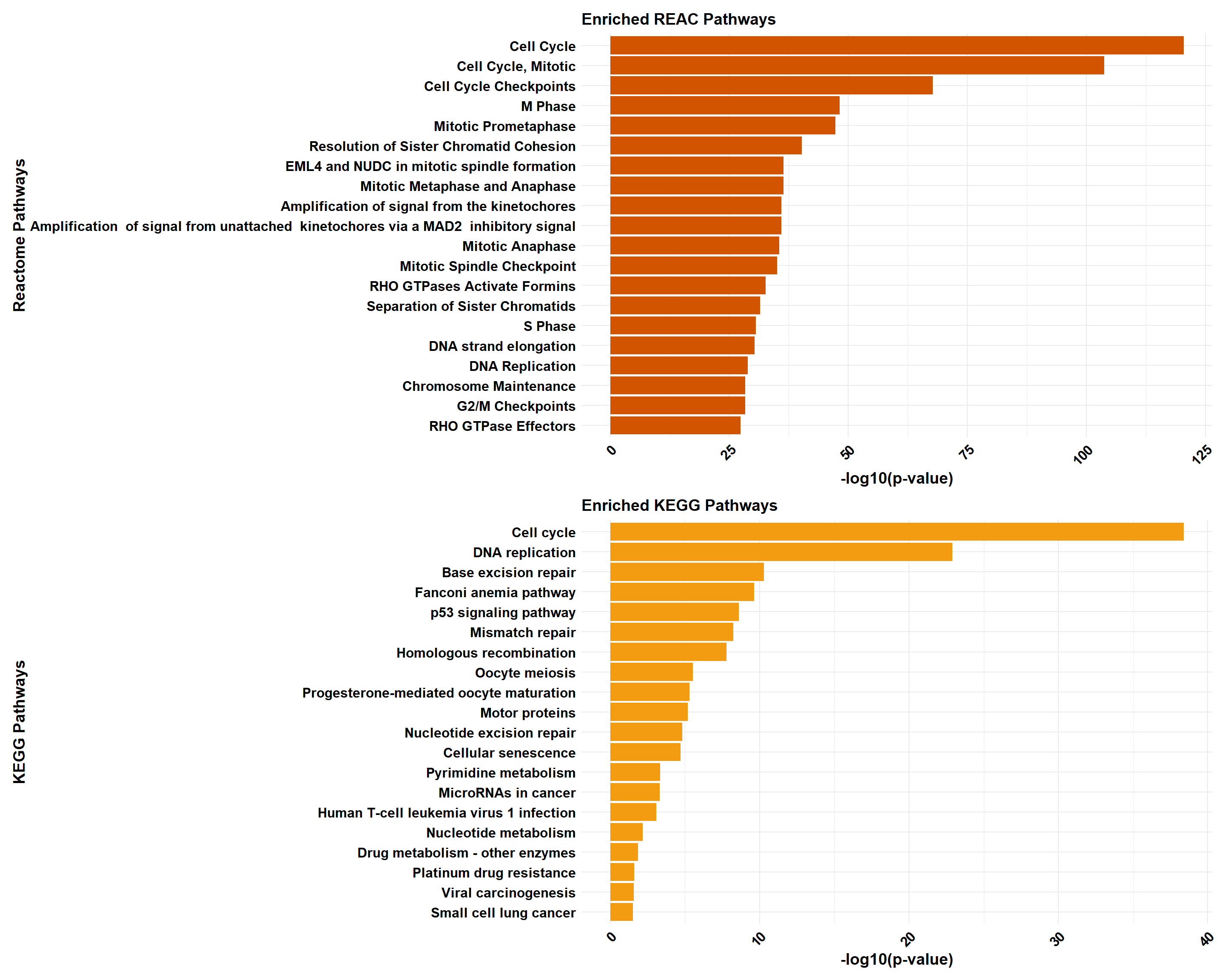
📌 DOX only mid-late (0.1 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load GO Enrichment CSV
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_DOX_only_mid-late_(0.1).csv")
# 🧠 Convert and filter (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# Create tibbles for each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plot function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🔧 Plot for each GO category
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")Warning in max(tibble$neglog, na.rm = TRUE): no non-missing arguments to max;
returning -Infplot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine plots
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot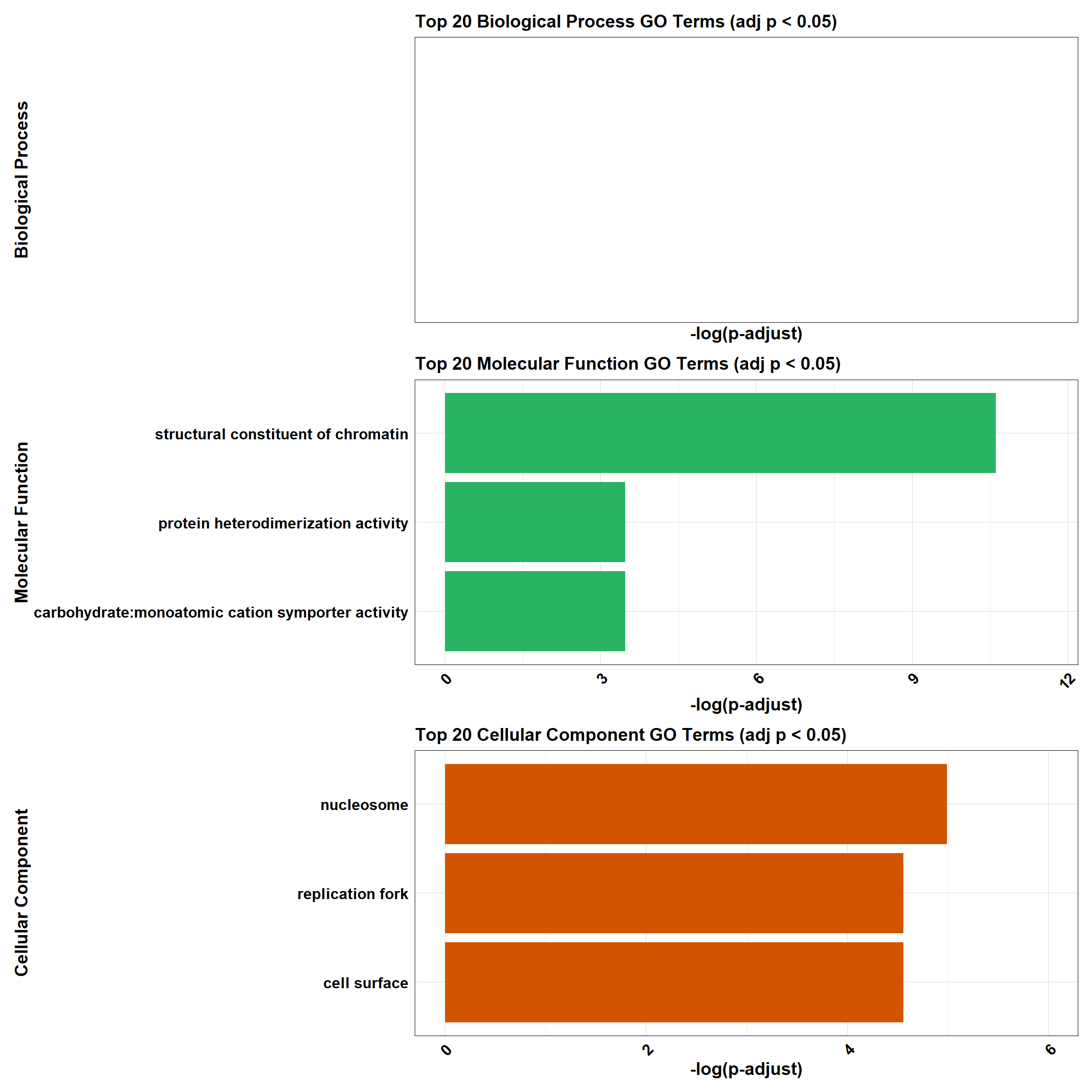
| Version | Author | Date |
|---|---|---|
| 6484005 | sayanpaul01 | 2025-02-27 |
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_3_0.1, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
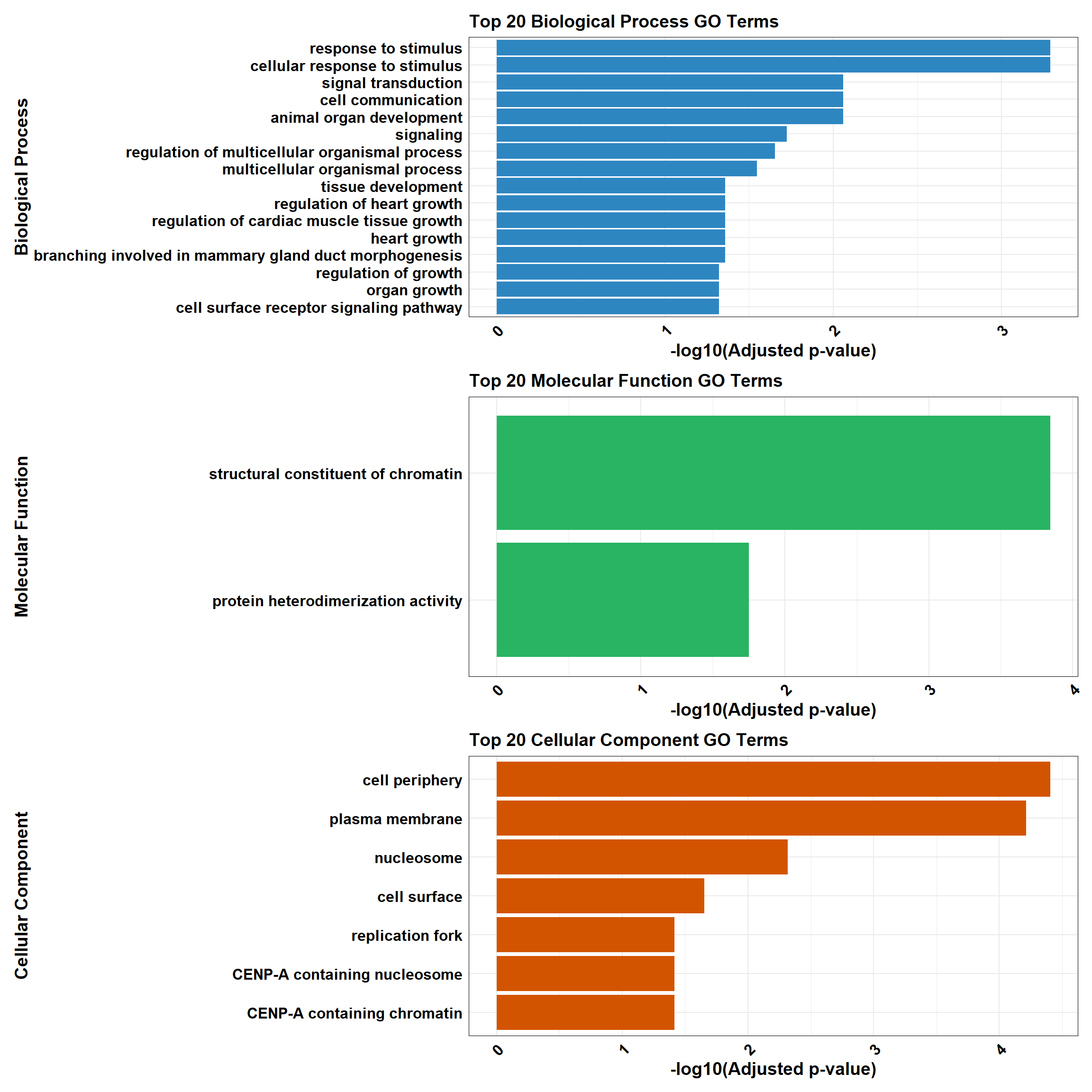
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_3_0.1,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_3_0.1,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_3_0.1,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_3_0.1,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)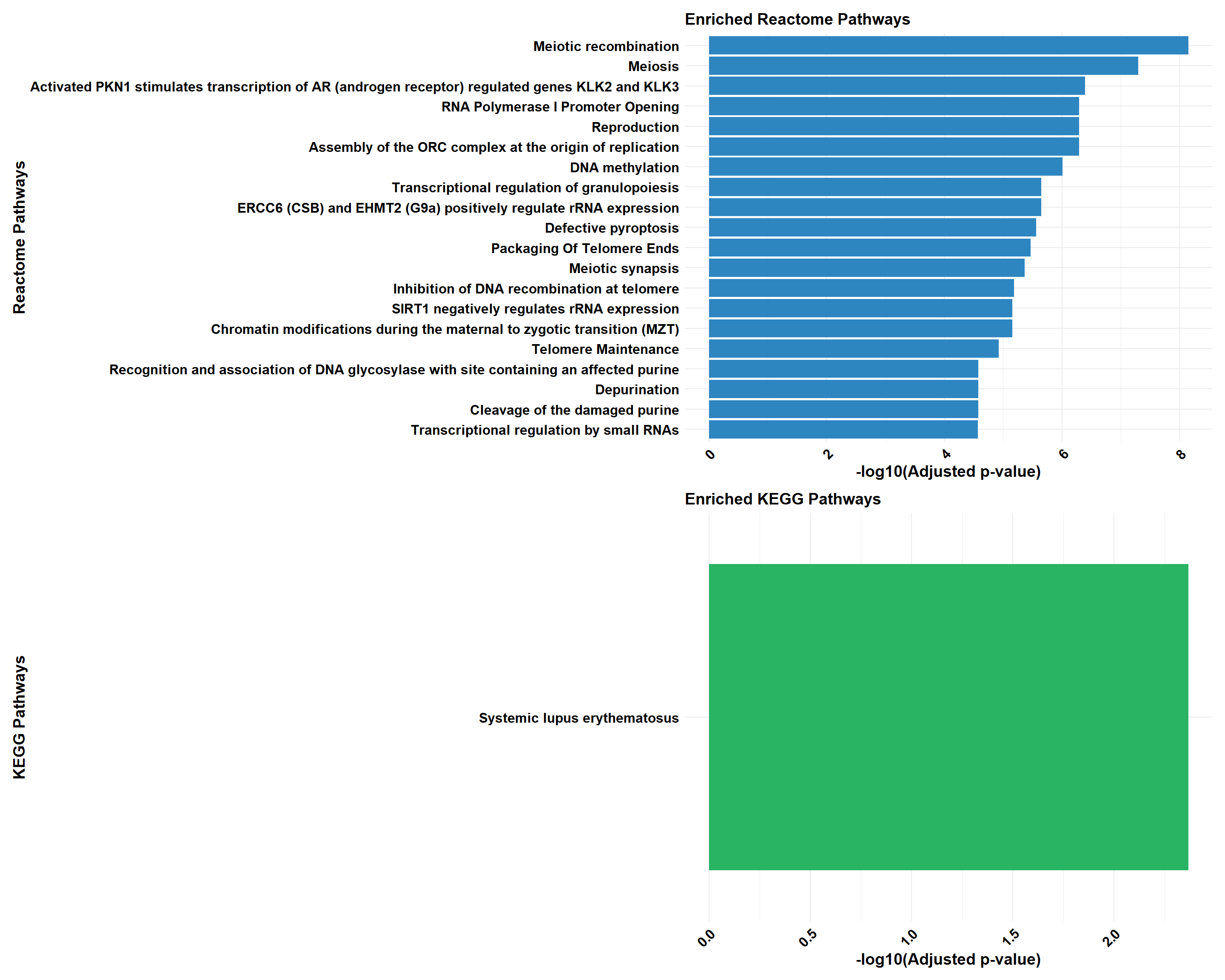
if (!is.null(gprofiler_combined)) print(gprofiler_combined)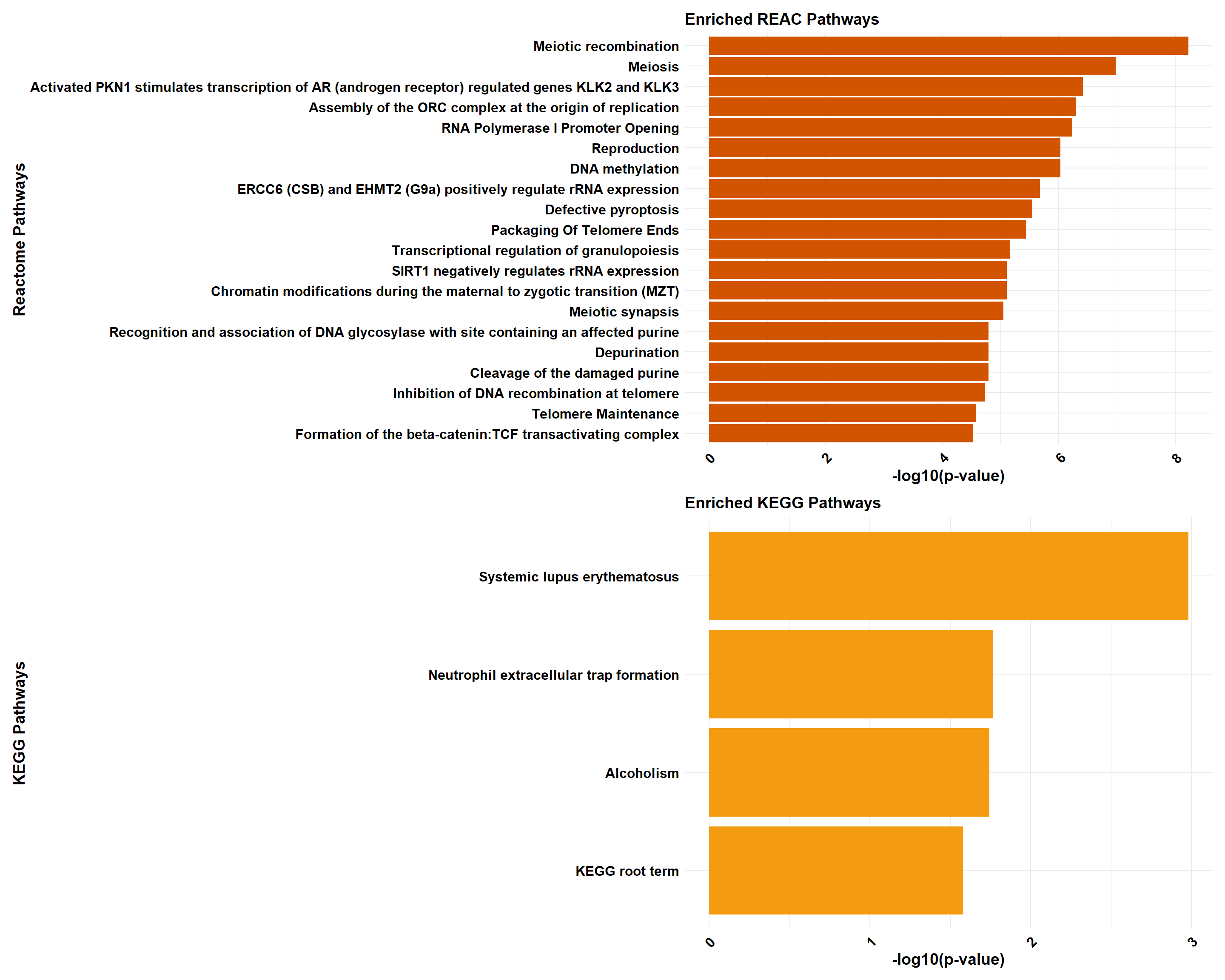
📌 0.5 Micromolar
📌 Read and Process Data
# Load the saved datasets
prob_1_0.5 <- read.csv("data/prob_1_0.5.csv")$Entrez_ID
prob_2_0.5 <- read.csv("data/prob_2_0.5.csv")$Entrez_ID
prob_3_0.5 <- read.csv("data/prob_3_0.5.csv")$Entrez_ID
prob_4_0.5 <- read.csv("data/prob_4_0.5.csv")$Entrez_ID
prob_5_0.5 <- read.csv("data/prob_5_0.5.csv")$Entrez_ID
CX_0.1_3 <- read.csv("data/DEGs/Toptable_CX_0.1_3.csv")
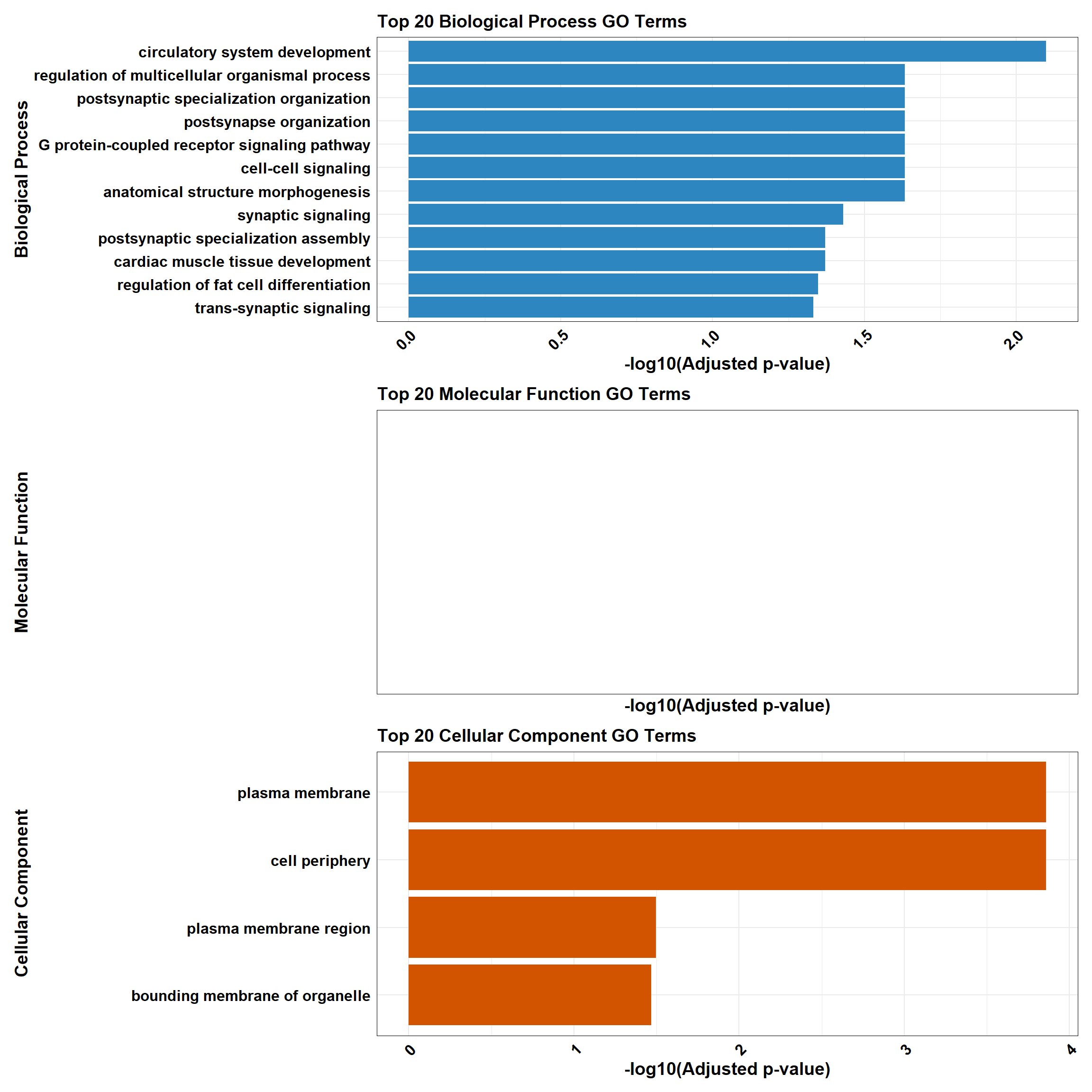
background<-as.character(CX_0.1_3$Entrez_ID)📌 Non response (0.5 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load Enrichment File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_Non_response_(0.5).csv")
# 🧠 Process Enrichment (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# ⛏️ Extract each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plotting Function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🎨 Generate GO Plots
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine & Show
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot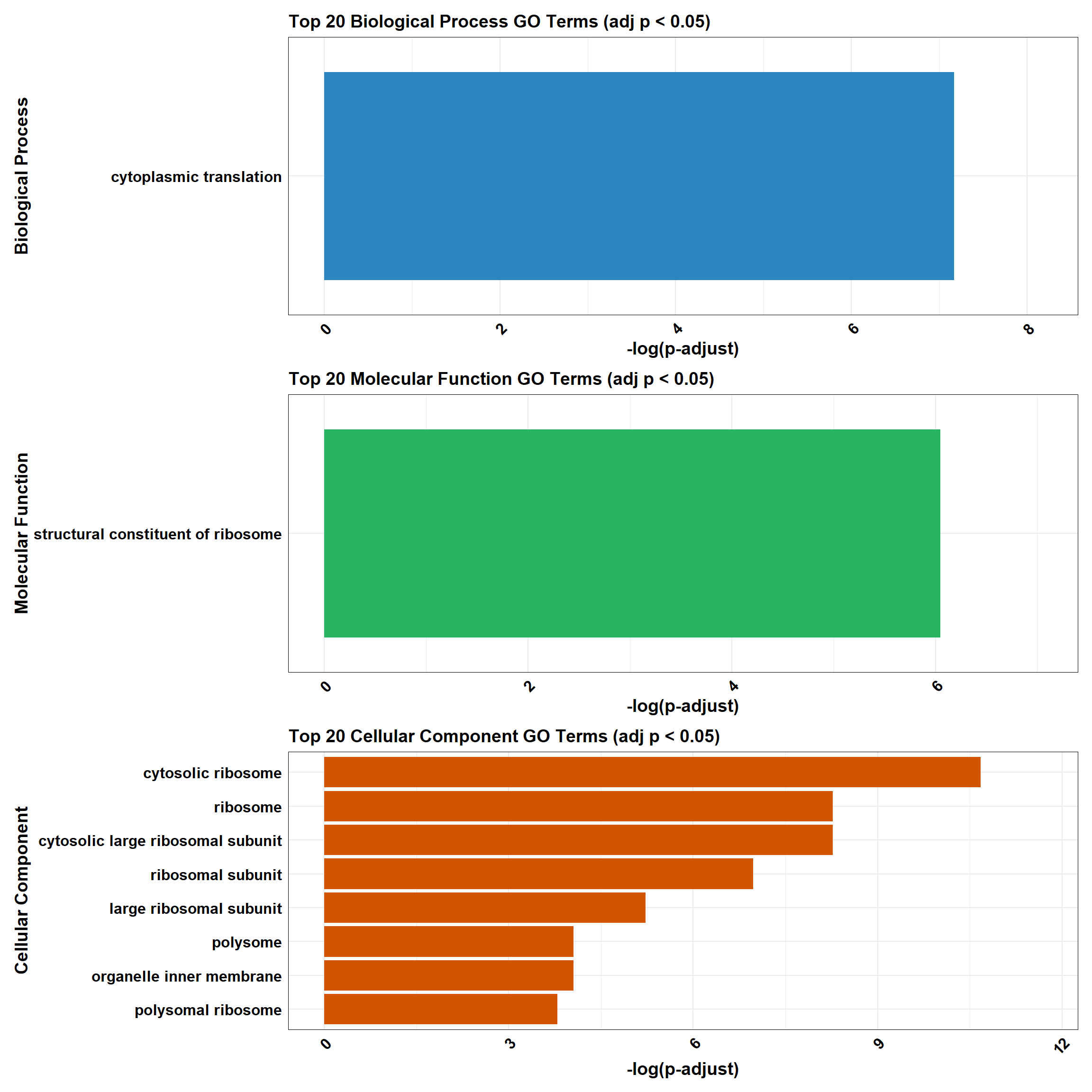
| Version | Author | Date |
|---|---|---|
| 11895c8 | sayanpaul01 | 2025-02-27 |
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
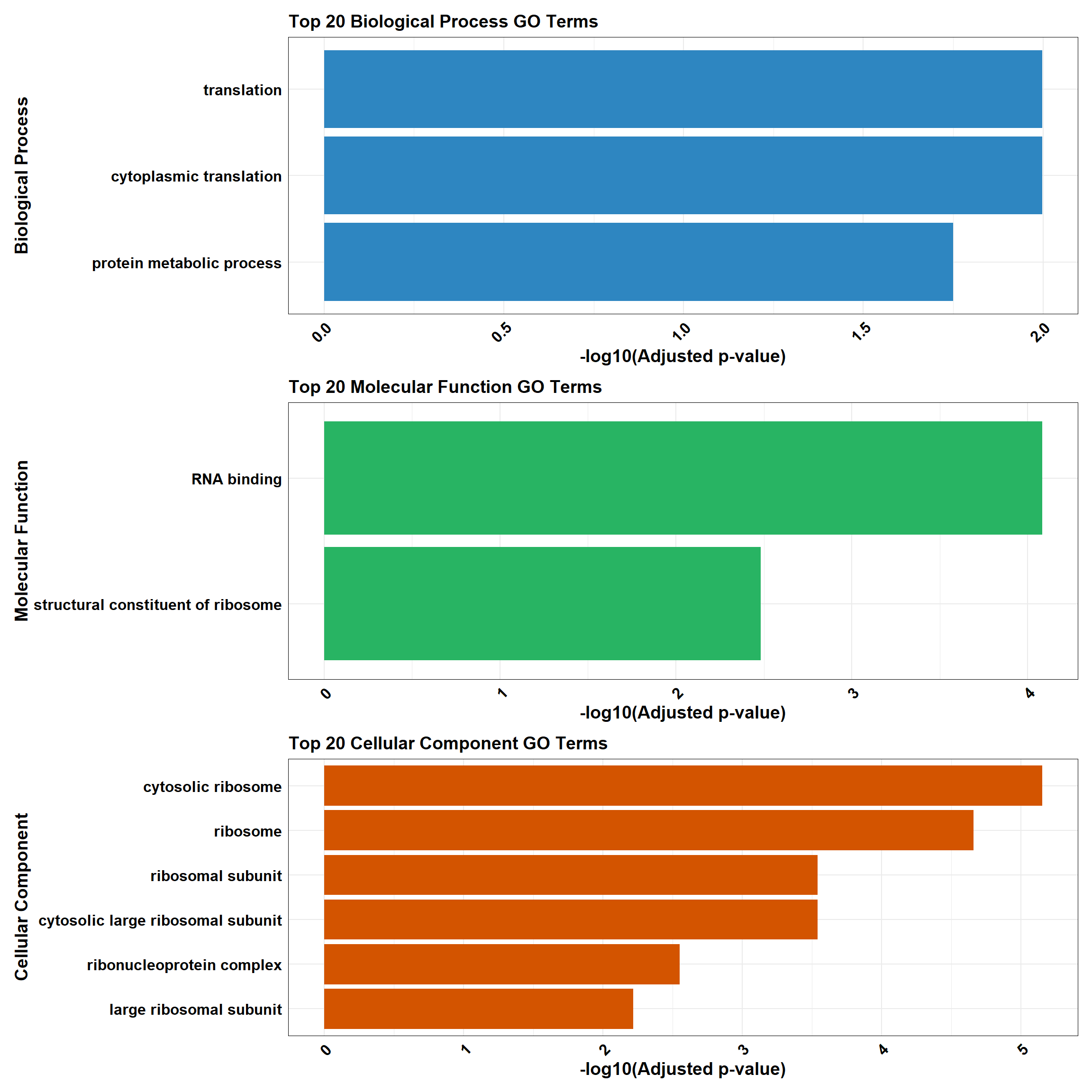
gost_results <- gost(
query = prob_1_0.5, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_1_0.5,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_1_0.5,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_1_0.5,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_1_0.5,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)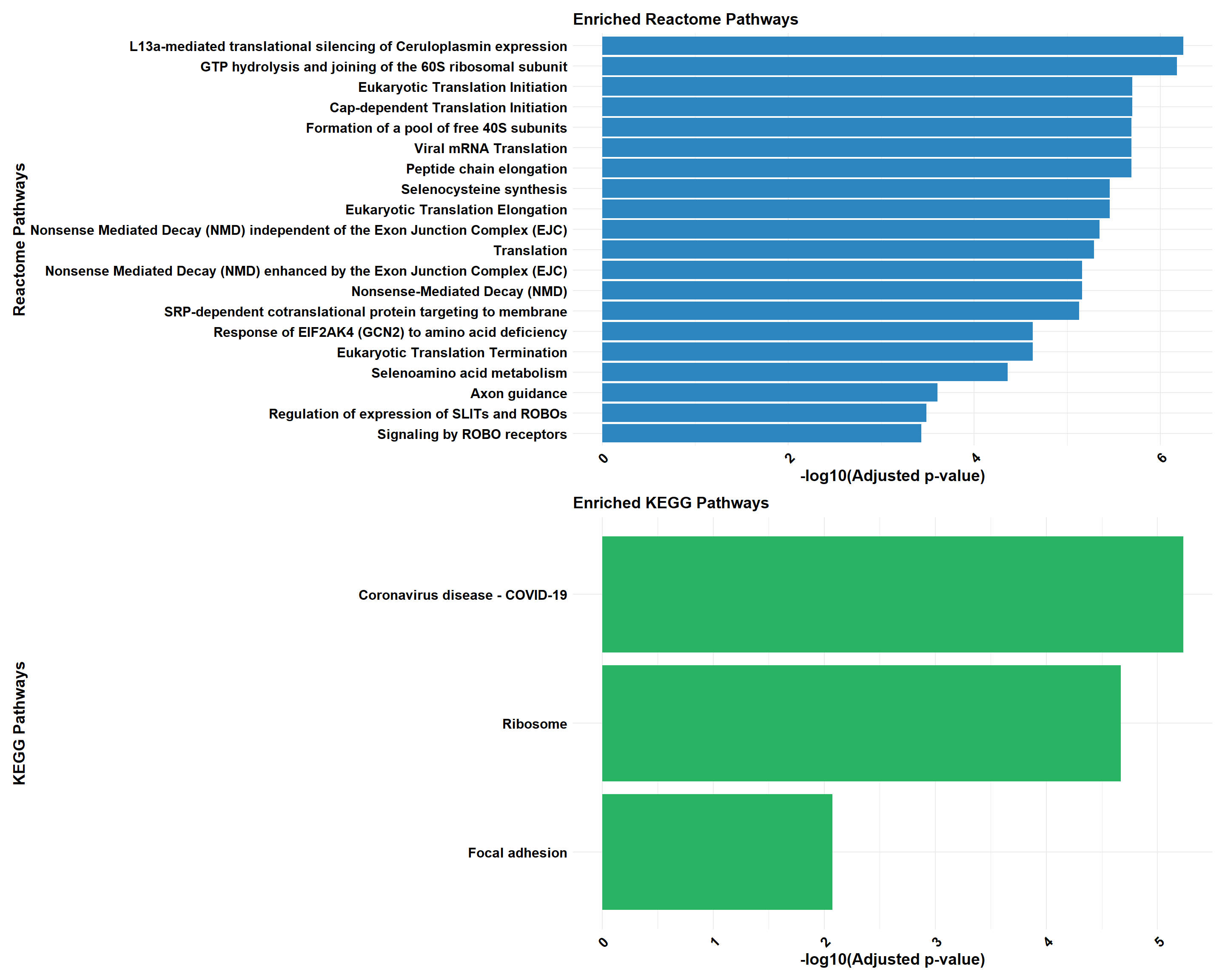
if (!is.null(gprofiler_combined)) print(gprofiler_combined)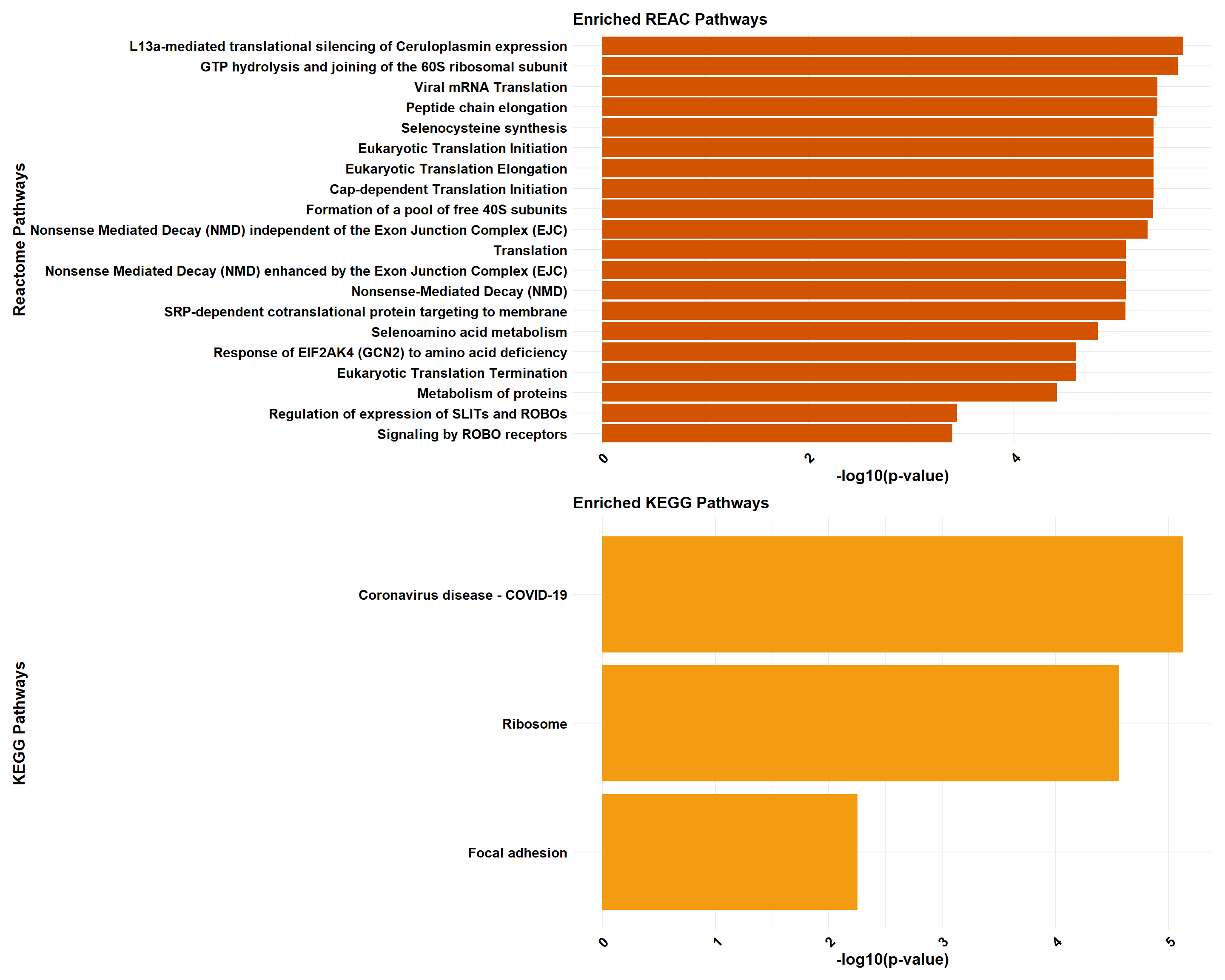
📌 DOX-specific response (0.5 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load Enrichment File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_DOX_specific_response_(0.5).csv")
# 🧠 Process Enrichment (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# ⛏️ Extract each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plotting Function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🎨 Generate GO Plots
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")Warning in max(tibble$neglog, na.rm = TRUE): no non-missing arguments to max;
returning -Infplot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine & Show
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot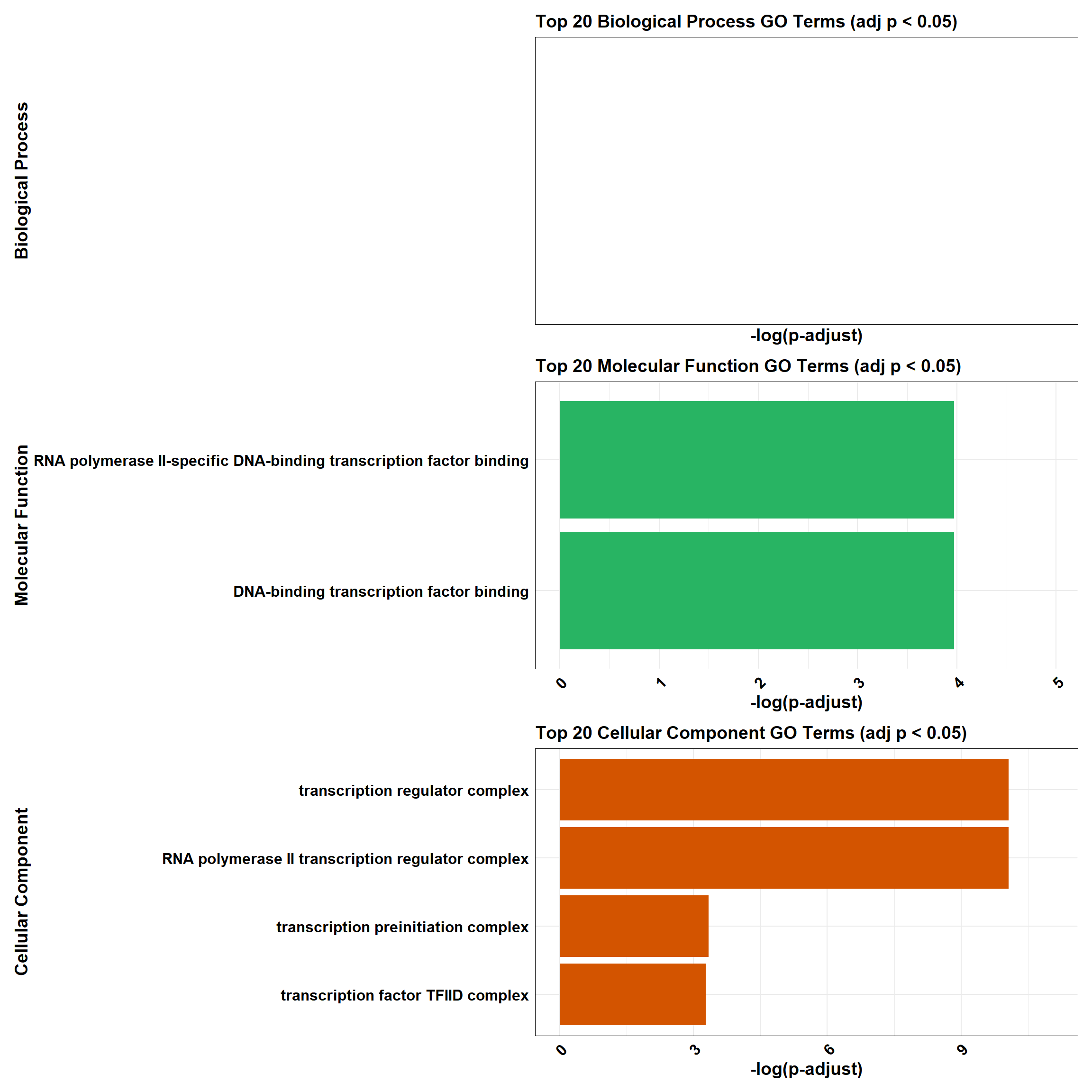
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_2_0.5, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_2_0.5,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_2_0.5,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_2_0.5,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_2_0.5,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)
if (!is.null(gprofiler_combined)) print(gprofiler_combined)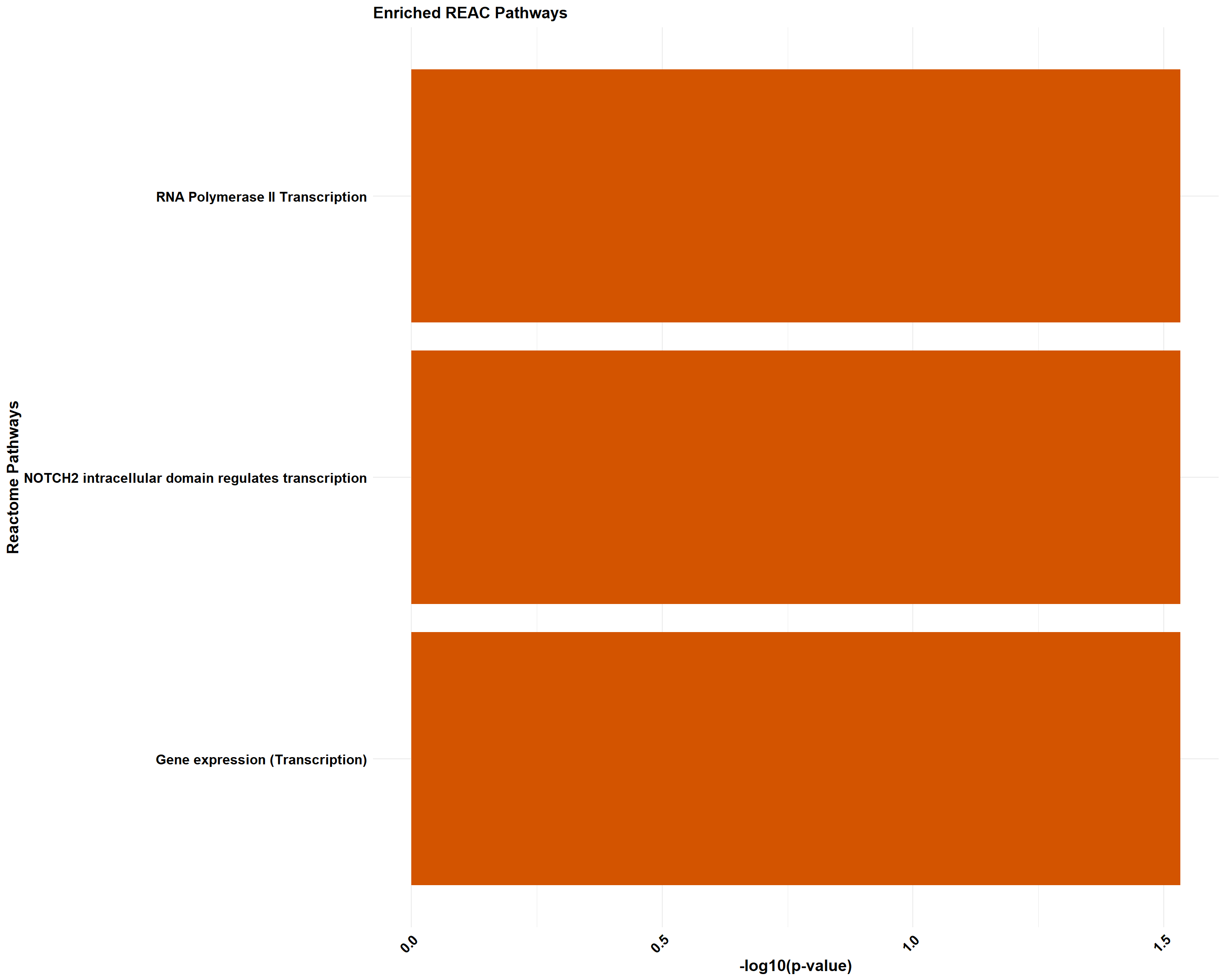
📌 DOX only mid-late response (0.5 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load Enrichment File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_DOX_only_mid-late_response_(0.5).csv")
# 🧠 Process Enrichment (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# ⛏️ Extract each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plotting Function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🎨 Generate GO Plots
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")Warning in max(tibble$neglog, na.rm = TRUE): no non-missing arguments to max;
returning -Infplot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")Warning in max(tibble$neglog, na.rm = TRUE): no non-missing arguments to max;
returning -Inf# 🧩 Combine & Show
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot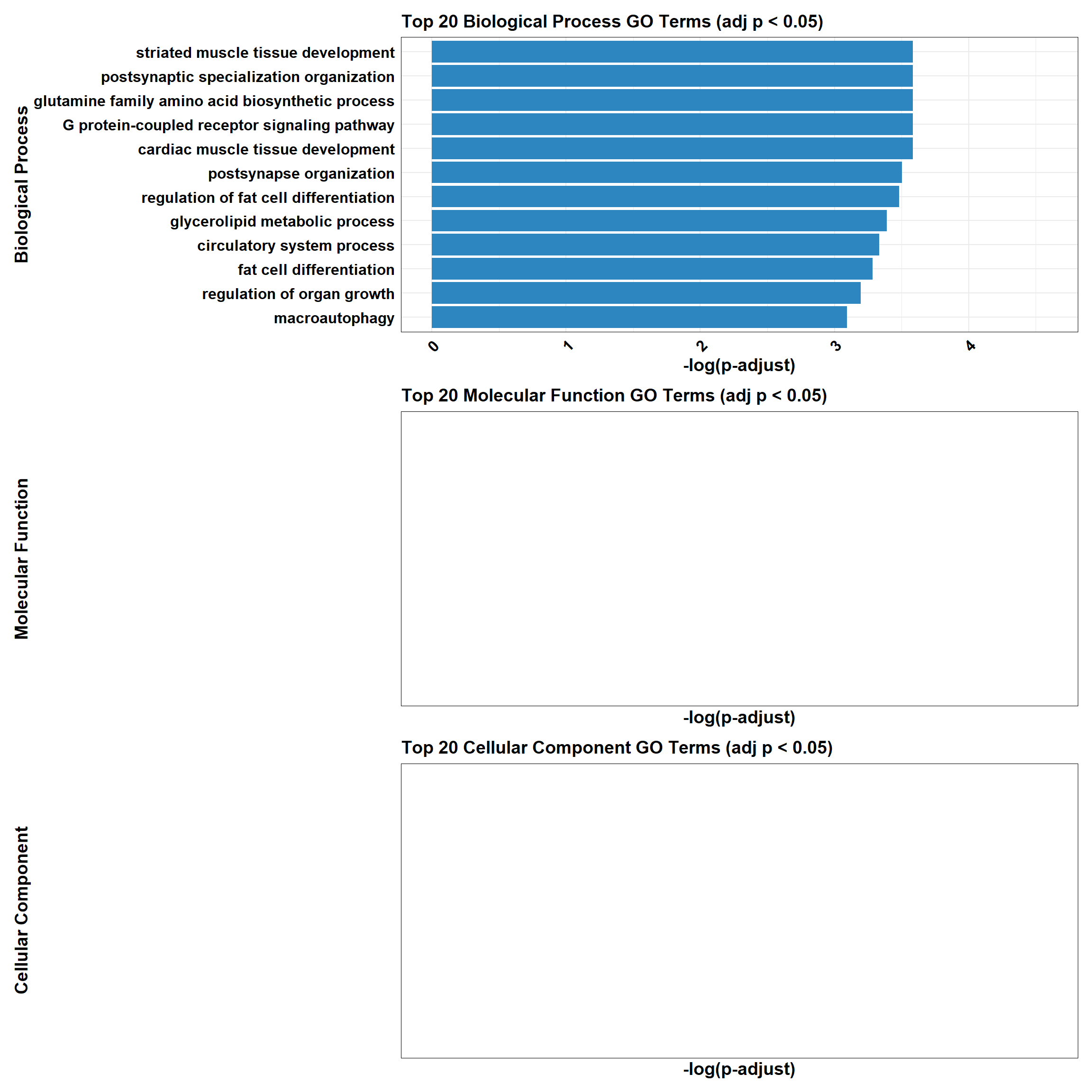
| Version | Author | Date |
|---|---|---|
| 11895c8 | sayanpaul01 | 2025-02-27 |
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_3_0.5, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_3_0.5,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_3_0.5,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_3_0.5,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_3_0.5,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)
if (!is.null(gprofiler_combined)) print(gprofiler_combined)📌 CX total + DOX early response (0.5 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load Enrichment File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_CX_total_+_DOX_early_response_(0.5).csv")
# 🧠 Process Enrichment (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# ⛏️ Extract each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plotting Function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🎨 Generate GO Plots
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine & Show
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot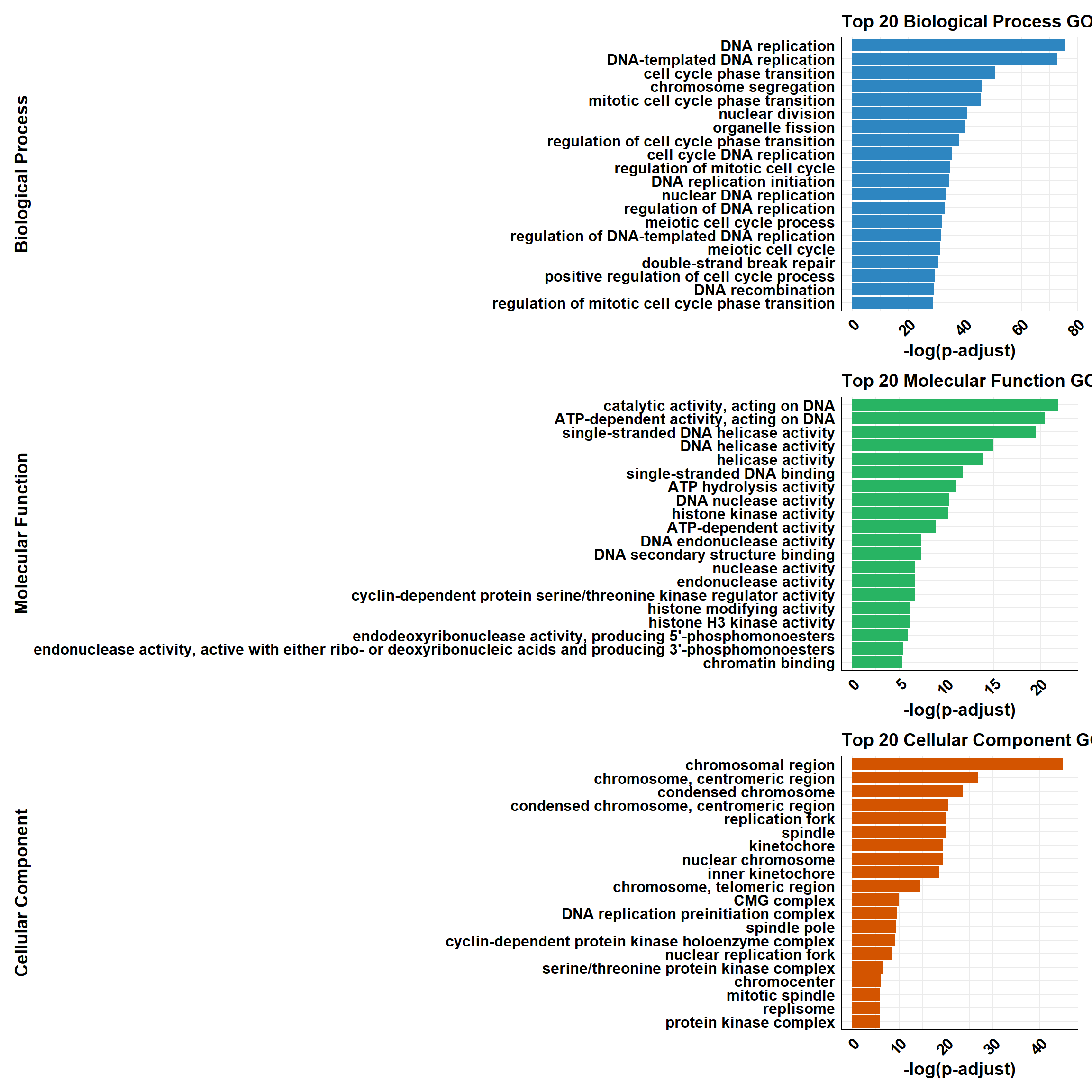
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_4_0.5, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}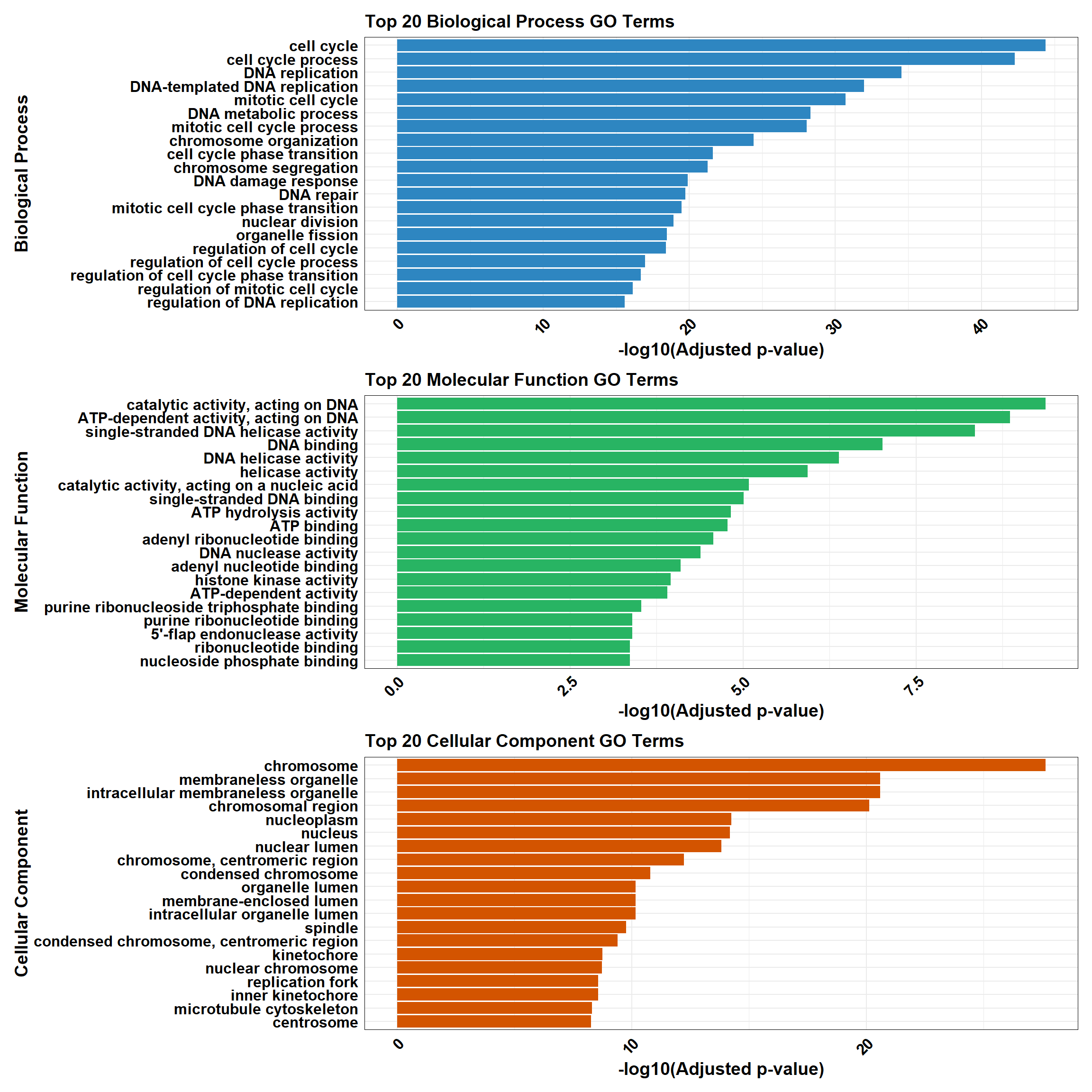
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_4_0.5,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_4_0.5,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_4_0.5,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_4_0.5,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)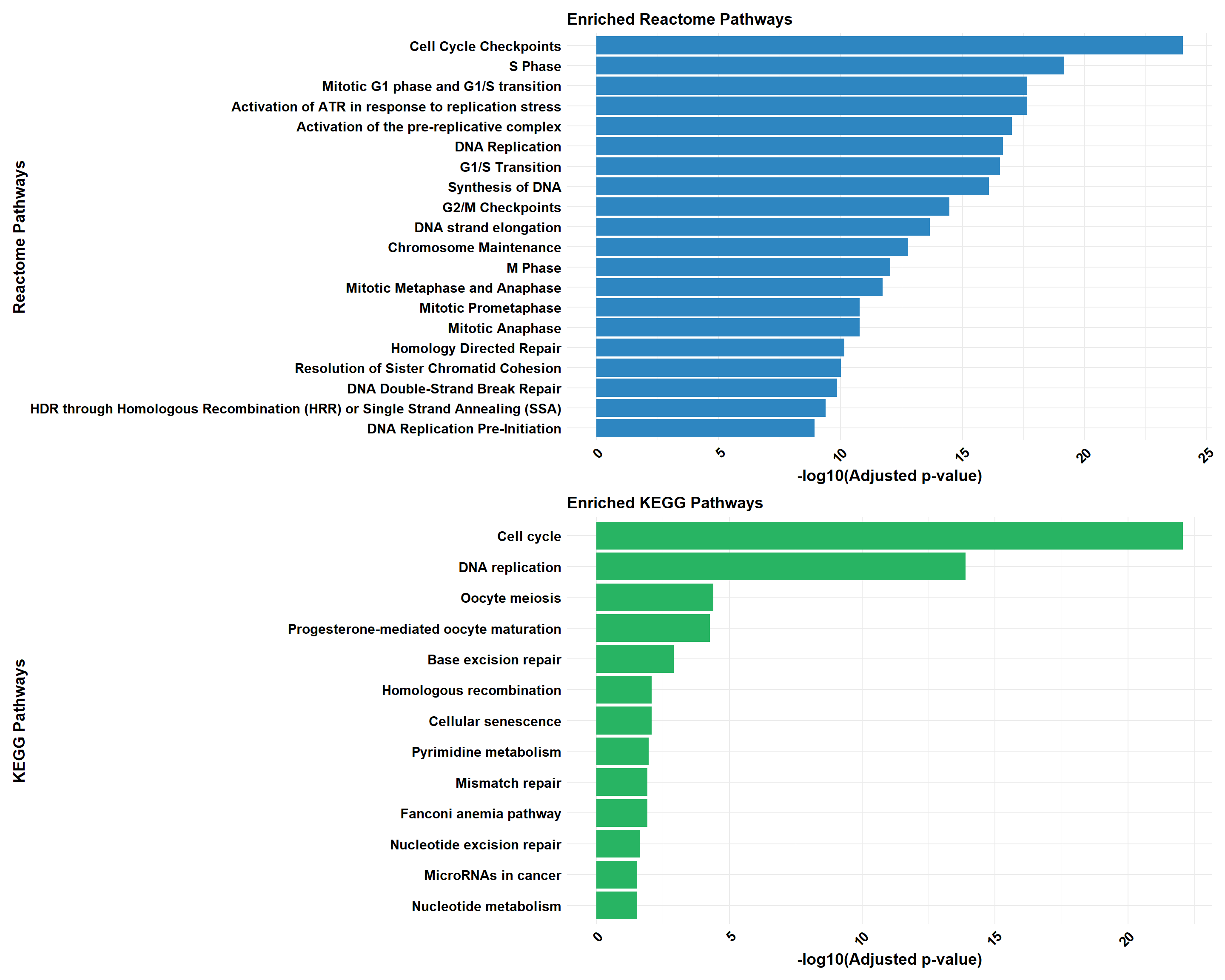
if (!is.null(gprofiler_combined)) print(gprofiler_combined)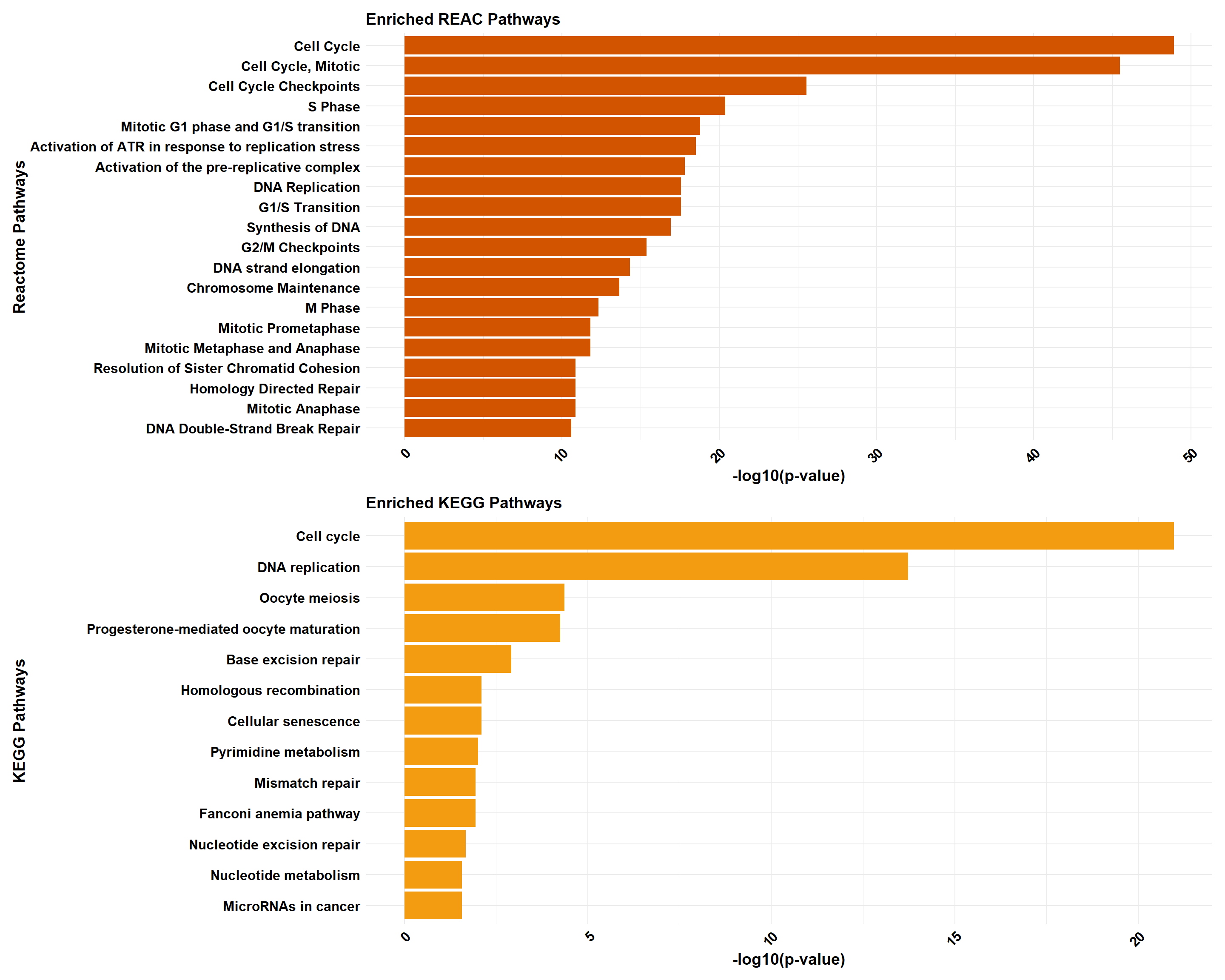
📌 DOX early + CX-DOX mid-late response (0.5 µM)
📌 GO Enrichment Clusterprofiler
# 📦 Load Required Libraries
library(tidyverse)
library(data.table)
library(patchwork)
# 📁 Load Enrichment File
go_df <- fread("data/BP/CorMotif_Terms_Combined/GO_All_DOX_early_+_CX-DOX_mid-late_response_(0.5).csv")
# 🧠 Process Enrichment (only adj p < 0.05)
process_enrichment_tibble <- function(df, ontology, category_label) {
df %>%
filter(Ontology == ontology & p.adjust < 0.05) %>%
mutate(neglog = -log(p.adjust)) %>%
arrange(desc(neglog)) %>%
slice(1:20) %>%
mutate(Category = category_label)
}
# ⛏️ Extract each GO class
BP_Tibble <- process_enrichment_tibble(go_df, "BP", "Biological Process")
MF_Tibble <- process_enrichment_tibble(go_df, "MF", "Molecular Function")
CC_Tibble <- process_enrichment_tibble(go_df, "CC", "Cellular Component")
# 📊 Plotting Function
process_enrichment_plot <- function(tibble, title, color) {
ggplot(tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log(p-adjust)", y = title,
title = paste("Top 20", title, "GO Terms (adj p < 0.05)")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, linewidth = 0.3)
) +
xlim(0, max(tibble$neglog, na.rm = TRUE) + 1)
}
# 🎨 Generate GO Plots
plot_BP <- process_enrichment_plot(BP_Tibble, "Biological Process", "#2E86C1")
plot_MF <- process_enrichment_plot(MF_Tibble, "Molecular Function", "#28B463")
plot_CC <- process_enrichment_plot(CC_Tibble, "Cellular Component", "#D35400")
# 🧩 Combine & Show
combined_plot <- plot_BP / plot_MF / plot_CC
combined_plot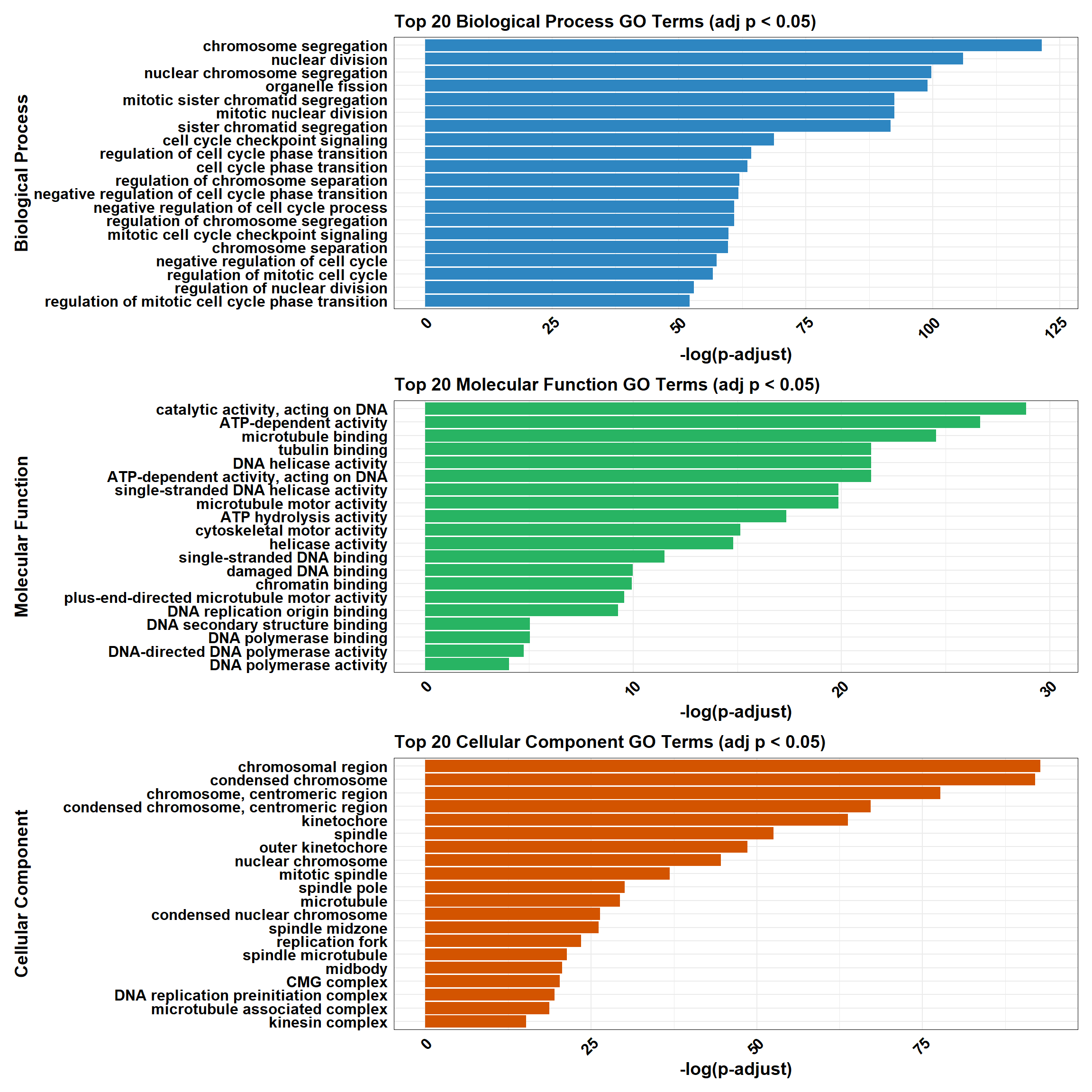
📌 GO Enrichment g:Profiler
# Load the gprofiler2 package
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
# Perform GO enrichment analysis with gprofiler2
gost_results <- gost(
query = prob_5_0.5, # List of input genes (prob_all_1)
organism = "hsapiens", # Human organism
user_threshold = 0.05, # Adjusted p-value cutoff
correction_method = "fdr", # Multiple testing correction
domain_scope = "custom", # Use custom background
custom_bg = background, # Background set of genes
sources = c("GO:BP", "GO:MF", "GO:CC") # Analyze GO categories
)
# Check if enrichment results exist
if (is.null(gost_results$result) || nrow(gost_results$result) == 0) {
# If no enriched terms, create a placeholder dataframe
combined_results <- tibble(
term_name = "No enriched terms",
p.adjust = NA,
source = "N/A",
Category = "N/A"
)
} else {
# Convert results to a data frame
gost_results_df <- gost_results$result
# Add a column for adjusted p-value
gost_results_df <- gost_results_df %>%
rename(p.adjust = p_value)
# Separate results for BP, MF, and CC
BP_results <- gost_results_df %>%
filter(source == "GO:BP") %>%
mutate(Category = "Biological Process")
MF_results <- gost_results_df %>%
filter(source == "GO:MF") %>%
mutate(Category = "Molecular Function")
CC_results <- gost_results_df %>%
filter(source == "GO:CC") %>%
mutate(Category = "Cellular Component")
# Select the top 20 terms by adjusted p-value for each category
top_BP <- BP_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_MF <- MF_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
top_CC <- CC_results %>%
arrange(p.adjust) %>%
slice_head(n = 20)
# Combine all categories
combined_results <- bind_rows(top_BP, top_MF, top_CC)
}
# Ensure all columns are atomic types for CSV export
combined_results_clean <- combined_results %>%
mutate(across(everything(), ~ if (is.list(.)) sapply(., toString) else .))
# Function for plotting top terms
plot_gprofiler_results <- function(data, title, color) {
ggplot(data, aes(x = -log10(p.adjust), y = reorder(term_name, -log10(p.adjust)))) +
geom_bar(stat = "identity", fill = color) +
labs(
x = "-log10(Adjusted p-value)",
y = title,
title = paste("Top 20", title, "GO Terms")
) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black"),
panel.border = element_rect(colour = "black", fill = NA, size = 0.3)
)
}
# Check if there are enrichment terms to plot
if (nrow(combined_results) == 1 && combined_results$term_name == "No enriched terms") {
message("No enriched GO terms found for the input gene set.")
} else {
# Plot the top 20 terms for each category
plot_BP <- plot_gprofiler_results(top_BP, "Biological Process", "#2E86C1")
plot_MF <- plot_gprofiler_results(top_MF, "Molecular Function", "#28B463")
plot_CC <- plot_gprofiler_results(top_CC, "Cellular Component", "#D35400")
# Combine the plots using patchwork
combined_plot <- plot_BP / plot_MF / plot_CC
# Display the combined plot
combined_plot
}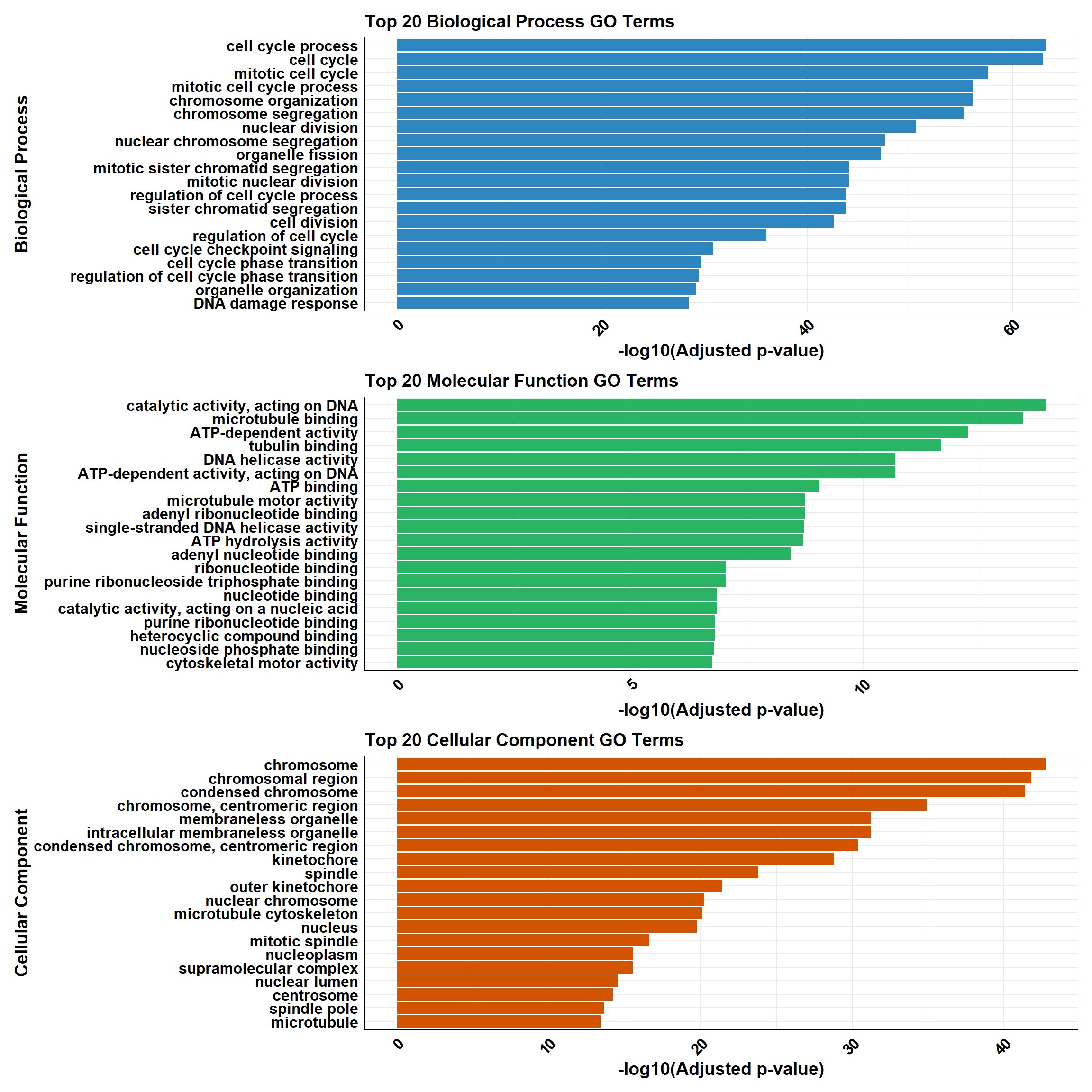
📌 Pathway Enrichment
# Load required libraries
library(clusterProfiler)
library(org.Hs.eg.db) # Required for enrichPathway
library(gprofiler2)
library(ggplot2)
library(dplyr)
library(patchwork)
library(ReactomePA)
# Function for ClusterProfiler Reactome & KEGG Analysis
process_clusterProfiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment based on the selected category
if (category == "Reactome") {
enrichment <- enrichPathway(
gene = gene_set,
organism = "human",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
} else if (category == "KEGG") {
enrichment <- enrichKEGG(
gene = gene_set,
organism = "hsa",
pvalueCutoff = 0.05,
pAdjustMethod = "BH",
universe = background
)
}
# Check if enrichment results exist
if (is.null(enrichment) || nrow(as.data.frame(enrichment)) == 0) {
message(paste("No significant enrichment found for", category, "in ClusterProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- as_tibble(as.data.frame(enrichment)) %>%
mutate(Category = category,
neglog = -log10(p.adjust)) %>% # Compute -log10(p.adjust)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(Description, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(Adjusted p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Function for gProfiler Reactome & KEGG Analysis
process_gprofiler <- function(gene_set, background, category, color, y_title) {
# Perform enrichment using gprofiler2
enrichment <- gost(
query = gene_set,
organism = "hsapiens",
user_threshold = 0.05,
correction_method = "fdr",
domain_scope = "custom",
custom_bg = background,
sources = category # Either "REAC" or "KEGG"
)
# Check if enrichment results exist
if (is.null(enrichment$result) || nrow(enrichment$result) == 0) {
message(paste("No significant enrichment found for", category, "in gProfiler"))
return(NULL)
}
# Convert results to tibble and process top 20 terms
enrichment_tibble <- enrichment$result %>%
as_tibble() %>%
mutate(Category = category,
neglog = -log10(p_value)) %>% # Compute -log10(p-value)
arrange(desc(neglog)) %>%
slice_head(n = min(20, nrow(.))) # Ensure safe slicing
# Generate plot
plot <- ggplot(enrichment_tibble, aes(x = neglog, y = reorder(term_name, neglog))) +
geom_bar(stat = "identity", fill = color) +
labs(x = "-log10(p-value)",
y = y_title,
title = paste("Enriched", category, "Pathways")) +
theme_minimal() +
theme(
axis.text.x = element_text(size = 12, face = "bold", colour = "black", angle = 45, hjust = 1),
axis.text.y = element_text(size = 12, face = "bold", colour = "black"),
axis.title.x = element_text(size = 14, face = "bold", colour = "black"),
axis.title.y = element_text(size = 14, face = "bold", colour = "black"),
plot.title = element_text(size = 14, face = "bold", colour = "black")
)
return(plot)
}
# Perform analysis for Reactome and KEGG using ClusterProfiler
cluster_reactome <- process_clusterProfiler(
gene_set = prob_5_0.5,
background = background,
category = "Reactome",
color = "#2E86C1",
y_title = "Reactome Pathways"
)
cluster_kegg <- process_clusterProfiler(
gene_set = prob_5_0.5,
background = background,
category = "KEGG",
color = "#28B463",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for ClusterProfiler
if (!is.null(cluster_reactome) && !is.null(cluster_kegg)) {
cluster_combined <- cluster_reactome / cluster_kegg
} else if (!is.null(cluster_reactome)) {
cluster_combined <- cluster_reactome
} else if (!is.null(cluster_kegg)) {
cluster_combined <- cluster_kegg
} else {
cluster_combined <- NULL
}
# Perform analysis for Reactome and KEGG using GProfiler
gprofiler_reactome <- process_gprofiler(
gene_set = prob_5_0.5,
background = background,
category = "REAC", # Corrected category for Reactome in gProfiler
color = "#D35400",
y_title = "Reactome Pathways"
)
gprofiler_kegg <- process_gprofiler(
gene_set = prob_5_0.5,
background = background,
category = "KEGG",
color = "#F39C12",
y_title = "KEGG Pathways"
)
# Combine Reactome and KEGG for GProfiler
if (!is.null(gprofiler_reactome) && !is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_reactome / gprofiler_kegg
} else if (!is.null(gprofiler_reactome)) {
gprofiler_combined <- gprofiler_reactome
} else if (!is.null(gprofiler_kegg)) {
gprofiler_combined <- gprofiler_kegg
} else {
gprofiler_combined <- NULL
}
# Display plots (if they are not NULL)
if (!is.null(cluster_combined)) print(cluster_combined)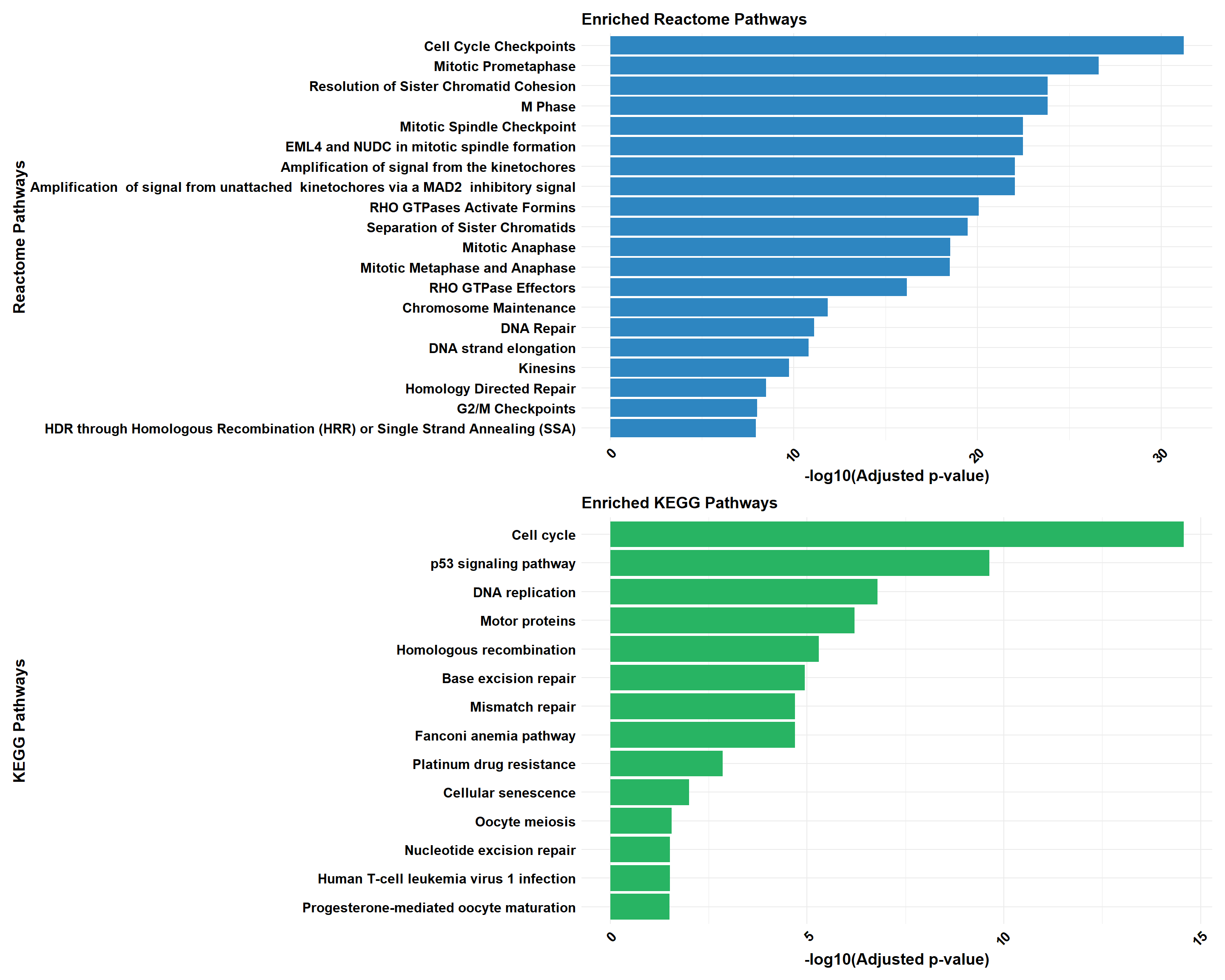
if (!is.null(gprofiler_combined)) print(gprofiler_combined)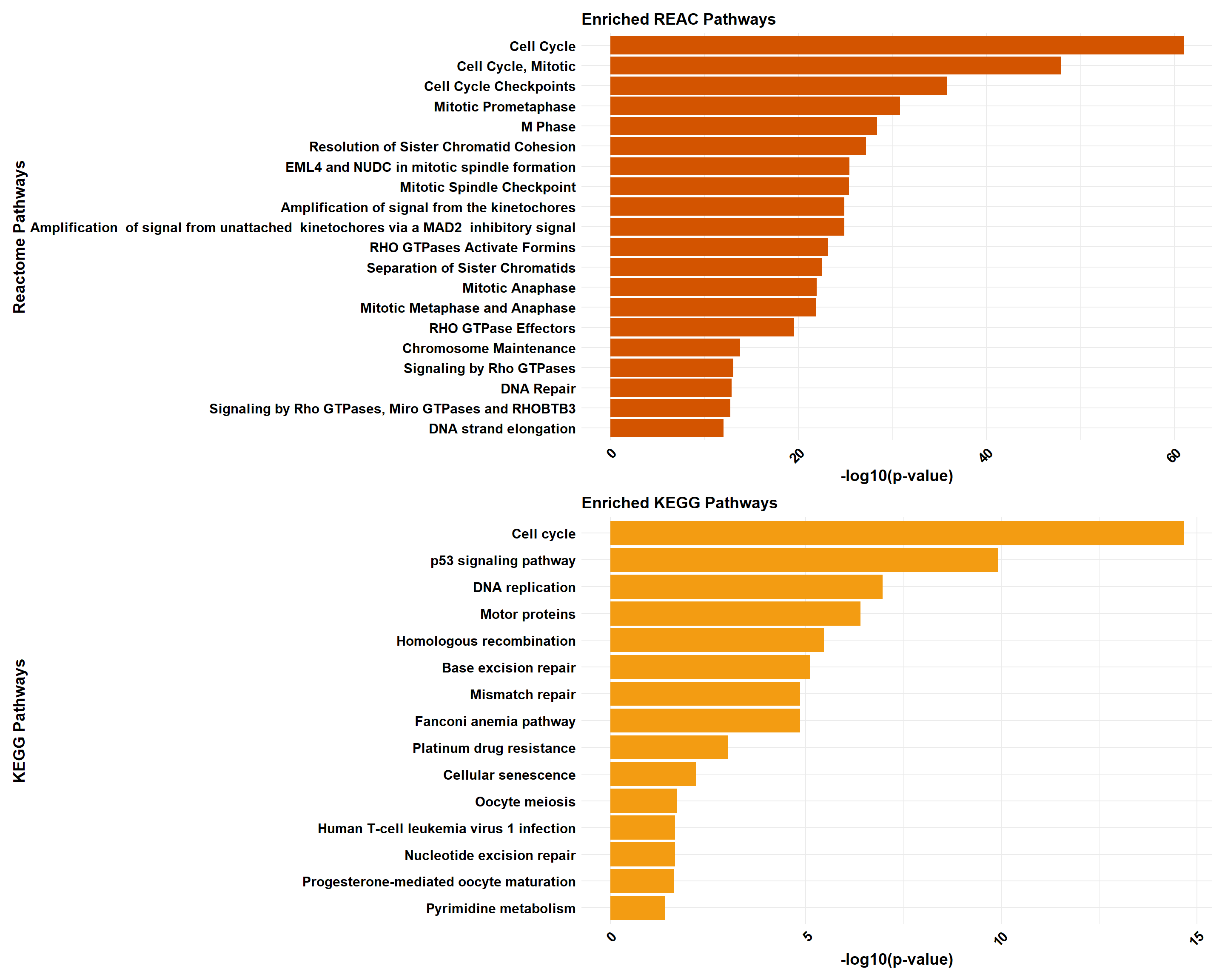
📌 Top BP (Cluster Profiler)
### 📦 Load Required Libraries
library(tidyverse)
library(ComplexHeatmap)Warning: package 'ComplexHeatmap' was built under R version 4.3.1library(circlize)Warning: package 'circlize' was built under R version 4.3.3library(grid)
### 📁 Define CorMotif GO Enrichment Files
go_files <- list(
"Non response (0.1)" = "data/BP/CorMotif_Terms/GO_BP_Non_response_(0.1).csv",
"CX-DOX mid-late response (0.1)" = "data/BP/CorMotif_Terms/GO_BP_CX-DOX_mid-late_response_(0.1).csv",
"DOX only mid-late (0.1)" = "data/BP/CorMotif_Terms/GO_BP_DOX_only_mid-late_(0.1).csv",
"Non response (0.5)" = "data/BP/CorMotif_Terms/GO_BP_Non_response_(0.5).csv",
"DOX specific response (0.5)" = "data/BP/CorMotif_Terms/GO_BP_DOX_specific_response_(0.5).csv",
"DOX only mid-late response (0.5)" = "data/BP/CorMotif_Terms/GO_BP_DOX_only_mid-late_response_(0.5).csv",
"CX total + DOX early response (0.5)" = "data/BP/CorMotif_Terms/GO_BP_CX_total_+_DOX_early_response_(0.5).csv",
"DOX early + CX-DOX mid-late response (0.5)" = "data/BP/CorMotif_Terms/GO_BP_DOX_early_+_CX-DOX_mid-late_response_(0.5).csv"
)
### 🔍 Step 1: Extract Top 5 GO Terms Per Group Based on Adjusted P-value
top_go_terms <- map(go_files, function(file) {
df <- tryCatch(read.csv(file), error = function(e) return(NULL))
if (!is.null(df) && nrow(df) > 0 && all(c("Description", "p.adjust") %in% colnames(df))) {
df %>%
as_tibble() %>%
filter(p.adjust < 0.05) %>%
arrange(p.adjust) %>%
dplyr::select(Description) %>%
slice_head(n = 5) %>%
pull(Description) %>%
unique()
} else {
character(0)
}
}) %>% unlist() %>% unique()
### 🔁 Step 2: Collect All Matrix Values for Top GO Terms
go_matrix_df <- map_dfr(names(go_files), function(group) {
file <- go_files[[group]]
df <- tryCatch(read.csv(file), error = function(e) return(tibble()))
if (nrow(df) == 0 || !all(c("Description", "pvalue", "p.adjust") %in% colnames(df))) {
tibble(Description = top_go_terms, pvalue = NA, p.adjust = NA, log10p = NA, Group = group)
} else {
df_filtered <- df %>%
as_tibble() %>%
dplyr::select(Description, pvalue, p.adjust) %>%
filter(Description %in% top_go_terms)
tibble(Description = top_go_terms) %>%
left_join(df_filtered, by = "Description") %>%
mutate(
log10p = ifelse(!is.na(pvalue), -log10(pvalue), NA),
Group = group
)
}
})
### 🧱 Step 3: Pivot to Heatmap Matrices
heatmap_data <- go_matrix_df %>%
dplyr::select(Description, Group, log10p) %>%
pivot_wider(names_from = Group, values_from = log10p) %>%
column_to_rownames("Description") %>%
as.matrix()
pval_matrix <- go_matrix_df %>%
dplyr::select(Description, Group, pvalue) %>%
pivot_wider(names_from = Group, values_from = pvalue) %>%
column_to_rownames("Description") %>%
as.matrix()
p_adj_matrix <- go_matrix_df %>%
dplyr::select(Description, Group, p.adjust) %>%
pivot_wider(names_from = Group, values_from = p.adjust) %>%
column_to_rownames("Description") %>%
as.matrix()
### 🎨 Step 4: Define Heatmap Color Gradient
breaks <- seq(0, 20, by = 2.5)
palette <- colorRampPalette(c("white", "#fde0dd", "#fa9fb5", "#f768a1", "#c51b8a", "#7a0177", "#49006a"))(length(breaks))
col_fun <- colorRamp2(breaks, palette)
### 🔥 Step 5: Draw Heatmap with Stars for p.adjust < 0.05
ht <- Heatmap(
heatmap_data,
name = "-log10(p)",
col = col_fun,
na_col = "white",
rect_gp = gpar(col = "black", lwd = 0.5),
cluster_rows = FALSE,
cluster_columns = FALSE,
row_names_gp = gpar(fontsize = 9),
column_names_gp = gpar(fontsize = 9),
column_names_rot = 45,
cell_fun = function(j, i, x, y, width, height, fill) {
adj_p <- p_adj_matrix[i, j]
if (!is.na(adj_p) && adj_p < 0.05) {
grid.text("*", x, y, gp = gpar(fontsize = 12))
}
},
heatmap_legend_param = list(
title = "-log10(p value)",
at = breaks,
labels = as.character(breaks),
legend_width = unit(5, "cm"),
direction = "horizontal",
title_gp = gpar(fontsize = 10, fontface = "bold"),
labels_gp = gpar(fontsize = 9)
)
)
### 🖼️ Final Output
draw(ht, heatmap_legend_side = "top")
sessionInfo()R version 4.3.0 (2023-04-21 ucrt)
Platform: x86_64-w64-mingw32/x64 (64-bit)
Running under: Windows 11 x64 (build 26100)
Matrix products: default
locale:
[1] LC_COLLATE=English_United States.utf8
[2] LC_CTYPE=English_United States.utf8
[3] LC_MONETARY=English_United States.utf8
[4] LC_NUMERIC=C
[5] LC_TIME=English_United States.utf8
time zone: America/Chicago
tzcode source: internal
attached base packages:
[1] grid stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] circlize_0.4.16
[2] ComplexHeatmap_2.18.0
[3] ReactomePA_1.46.0
[4] gprofiler2_0.2.3
[5] data.table_1.17.0
[6] patchwork_1.3.0
[7] DOSE_3.28.2
[8] TxDb.Hsapiens.UCSC.hg38.knownGene_3.18.0
[9] RColorBrewer_1.1-3
[10] clusterProfiler_4.10.1
[11] pheatmap_1.0.12
[12] qvalue_2.34.0
[13] BiocParallel_1.36.0
[14] Homo.sapiens_1.3.1
[15] TxDb.Hsapiens.UCSC.hg19.knownGene_3.2.2
[16] org.Hs.eg.db_3.18.0
[17] GO.db_3.18.0
[18] OrganismDbi_1.44.0
[19] GenomicFeatures_1.54.4
[20] GenomicRanges_1.54.1
[21] GenomeInfoDb_1.38.8
[22] AnnotationDbi_1.64.1
[23] IRanges_2.36.0
[24] S4Vectors_0.40.2
[25] Biobase_2.62.0
[26] BiocGenerics_0.48.1
[27] edgeR_4.0.16
[28] limma_3.58.1
[29] cluster_2.1.8.1
[30] ggfortify_0.4.17
[31] lubridate_1.9.4
[32] forcats_1.0.0
[33] stringr_1.5.1
[34] dplyr_1.1.4
[35] purrr_1.0.4
[36] readr_2.1.5
[37] tidyr_1.3.1
[38] tibble_3.2.1
[39] ggplot2_3.5.2
[40] tidyverse_2.0.0
loaded via a namespace (and not attached):
[1] splines_4.3.0 later_1.3.2
[3] BiocIO_1.12.0 bitops_1.0-9
[5] ggplotify_0.1.2 filelock_1.0.3
[7] polyclip_1.10-7 graph_1.80.0
[9] XML_3.99-0.18 lifecycle_1.0.4
[11] doParallel_1.0.17 rprojroot_2.0.4
[13] lattice_0.22-7 MASS_7.3-60
[15] magrittr_2.0.3 plotly_4.10.4
[17] sass_0.4.10 rmarkdown_2.29
[19] jquerylib_0.1.4 yaml_2.3.10
[21] httpuv_1.6.15 cowplot_1.1.3
[23] DBI_1.2.3 abind_1.4-8
[25] zlibbioc_1.48.2 ggraph_2.2.1
[27] RCurl_1.98-1.17 yulab.utils_0.2.0
[29] tweenr_2.0.3 rappdirs_0.3.3
[31] git2r_0.36.2 GenomeInfoDbData_1.2.11
[33] enrichplot_1.22.0 ggrepel_0.9.6
[35] tidytree_0.4.6 reactome.db_1.86.2
[37] codetools_0.2-20 DelayedArray_0.28.0
[39] xml2_1.3.8 ggforce_0.4.2
[41] shape_1.4.6.1 tidyselect_1.2.1
[43] aplot_0.2.5 farver_2.1.2
[45] viridis_0.6.5 matrixStats_1.5.0
[47] BiocFileCache_2.10.2 GenomicAlignments_1.38.2
[49] jsonlite_2.0.0 GetoptLong_1.0.5
[51] tidygraph_1.3.1 iterators_1.0.14
[53] foreach_1.5.2 tools_4.3.0
[55] progress_1.2.3 treeio_1.26.0
[57] Rcpp_1.0.12 glue_1.7.0
[59] gridExtra_2.3 SparseArray_1.2.4
[61] xfun_0.52 MatrixGenerics_1.14.0
[63] withr_3.0.2 BiocManager_1.30.25
[65] fastmap_1.2.0 digest_0.6.34
[67] timechange_0.3.0 R6_2.6.1
[69] gridGraphics_0.5-1 colorspace_2.1-0
[71] Cairo_1.6-2 biomaRt_2.58.2
[73] RSQLite_2.3.9 generics_0.1.3
[75] rtracklayer_1.62.0 htmlwidgets_1.6.4
[77] prettyunits_1.2.0 graphlayouts_1.2.2
[79] httr_1.4.7 S4Arrays_1.2.1
[81] scatterpie_0.2.4 graphite_1.48.0
[83] whisker_0.4.1 pkgconfig_2.0.3
[85] gtable_0.3.6 blob_1.2.4
[87] workflowr_1.7.1 XVector_0.42.0
[89] shadowtext_0.1.4 htmltools_0.5.8.1
[91] fgsea_1.28.0 RBGL_1.78.0
[93] clue_0.3-66 scales_1.3.0
[95] png_0.1-8 ggfun_0.1.8
[97] knitr_1.50 rstudioapi_0.17.1
[99] tzdb_0.5.0 reshape2_1.4.4
[101] rjson_0.2.23 nlme_3.1-168
[103] curl_6.2.2 GlobalOptions_0.1.2
[105] cachem_1.1.0 parallel_4.3.0
[107] HDO.db_0.99.1 restfulr_0.0.15
[109] pillar_1.10.2 vctrs_0.6.5
[111] promises_1.3.2 dbplyr_2.5.0
[113] evaluate_1.0.3 magick_2.8.6
[115] cli_3.6.1 locfit_1.5-9.12
[117] compiler_4.3.0 Rsamtools_2.18.0
[119] rlang_1.1.3 crayon_1.5.3
[121] labeling_0.4.3 plyr_1.8.9
[123] fs_1.6.3 stringi_1.8.3
[125] viridisLite_0.4.2 munsell_0.5.1
[127] Biostrings_2.70.3 lazyeval_0.2.2
[129] GOSemSim_2.28.1 Matrix_1.6-1.1
[131] hms_1.1.3 bit64_4.6.0-1
[133] KEGGREST_1.42.0 statmod_1.5.0
[135] SummarizedExperiment_1.32.0 igraph_2.1.4
[137] memoise_2.0.1 bslib_0.9.0
[139] ggtree_3.10.1 fastmatch_1.1-6
[141] bit_4.6.0 gson_0.1.0
[143] ape_5.8-1